Combined Educational & Scientific Session
Amyloid-Related Imaging Abnormalities
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 6 | 14:00 - 16:00 | Moderators: Giuseppe Barisano & Nivedita Agarwal |
 |
Amyloid-Related Imaging Abnormalities: What Are They?
Fabrizio Piazza
This lecture aims to gain an understanding of ARIA-E and ARIA-H, including identification, risk factors, clinical relevance, management, and the theoretical and practical implications of the proposed ARIA PARADOX model.
|
|
| Cerebral Amyloid Angiopathy: failure of intramural periarterial drainage?
Roxana Carare
|
||
 |
Imaging Biomarkers of Cerebral Amyloid Angiopathy Video Permission Withheld
Steven Greenberg
Cerebral amyloid angiopathy (CAA), deposition of ß-amyloid in small cortical and leptomeningeal vessels, is a common age-related pathology associated with lobar hemorrhage and cognitive impairment. Among MRI-based markers of CAA are lobar hemorrhages and microbleeds, convexity subarachnoid hemorrhage and cortical superficial siderosis, microinfarcts, altered structural connectivity on diffusion-tensor imaging, dilated perivascular spaces in centrum semiovale, multi-spot pattern white matter hyperintensities, and impaired vascular reactivity to visual stimulation. Current diagnosis of CAA per the Boston Criteria depends on detection of multiple strictly lobar hemorrhagic lesions. Ongoing studies suggest that incorporation of emerging white matter markers may improve sensitivity without compromising specificity.
|
|
| ARIA in Alzheimer Clinical Trials
Clifford Jack, Jr.
|
| Concurrent 6 | 14:00 - 16:00 | Moderators: |
| 0321. | Cortical ß-amyloid plaque load detection using QSM in Alzheimer’s patients at 9.4T
Elisa Tuzzi1,2, Rolf Pohmann3, Alexander Loktyushin3, Christoph Laske4,5, Klaus Scheffler3,6, and Gisela Elisabeth Hagberg3,6
1Department for Biomedical Magnetic Resonance, Eberhard Karl’s University and University Hospital, Tuebingen, Germany, 2Department for High Field Magnetic Resonance, Max Planck Institute for Biological Cybernetic, Tuebingen, Germany, 3Department for High Field Magnetic Resonance, Max Planck Institute for Biological Cybernetics, Tuebingen, Germany, 4German Center for Neurodegenerative Diseases (DZNE), Tuebingen, Germany, 5Section for Dementia Research, Hertie Institute for Clinical Brain Research and Department of Psychiatry and Psychotherapy, Tuebingen, Germany, 6Department for Biomedical Magnetic Resonance, Eberhard Karl’s University, Tuebingen and University Hospital, Tuebingen, Germany
Beta-amyloid (Aβ) plaques are characteristic of Alzheimer’s Disease (AD) brain and cause effects which can be detected by QSM. It has been shown that cortical plaque-load could be used to distinguish AD patients from healthy controls (HC) using ultra-high spatial resolution QSM at ultra-high-field (9.4 and 14.1T), in-vivo and ex-vivo. We aimed to extend these observations to a larger cohort of patients and controls at two different spatial resolutions. We found a significative (p<0.05) increase in plaque-load in AD compared to HC at both resolutions. Interestingly, some cortical regions also showed greater (p<0.05) diamagnetic effects in AD compared to HC.
|
||
0322.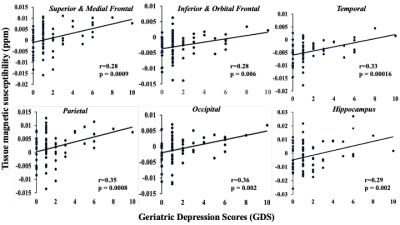 |
Iron deposits estimated by QSM MRI: a biomarker of depressive symptoms in cognitively normal and impaired adults with vascular problems
Sandeepa Sur1,2, Lin Chen1, Danyang Yu3, Leah H Rubin2,4, Yanxun H Xu5, Zixuan Lu1, Sevil Yasar6, Paul Rosenberg7, Rita Kalyani6, Kaisha H HAzel1, George H Pottanat8, Peter van Zijl9, Marilyn Albert2, Hanzhang Lu1,
and Xu Li9
1Radiology, Johns Hopkins University, Baltimore, MD, United States, 2Neurology, Johns Hopkins University, Baltimore, MD, United States, 3Engineering, Johns Hopkins University, Baltimore, MD, United States, 4Epidemiology, Johns Hopkins University, Baltimore, MD, United States, 5Whitting School of Engineering, Johns Hopkins University, Baltimore, MD, United States, 6Medicine, Johns Hopkins University, Baltimore, MD, United States, 7Psychiatry and Behavorial Sciences, Johns Hopkins University, Baltimore, MD, United States, 8Johns Hopkins University, Baltimore, MD, United States, 9Kirby Center, Kennedy Krieger Institute, Baltimore, MD, United States
This study explored whether brain iron deposits in gray matter, measured as increased magnetic susceptibility, is a good biomarker for depressive symptoms in older adults with normal and impaired cognition, and vascular comorbidities. In a cross-sectional study(n=73) of normal, mild-cognitive-impairment (MCI), and mild-dementia participants with vascular comorbidities, increased susceptibility in brain-regions (frontal, temporal, parietal, occipital, hippocampus and thalamus) was associated with depressive symptoms (Geriatric Depression Scale, GDS) after adjusting for age, sex, diagnosis, and structural-volume loss, suggesting its potential use as biomarker for depressive symptoms in normal and impaired older adults.
|
||
0323.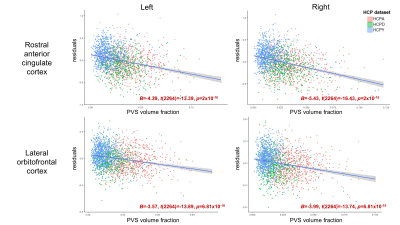 |
PVS enlargement preferentially influences regional cortical thickness across the normative lifespan
Kirsten Mary Lynch1, Arthur W Toga1, and Farshid Sepehrband1
1USC Mark and Mary Stevens Institute for Neuroimaging and Informatics, USC Keck School of Medicine, Los Angeles, CA, United States
Perivascular spaces (PVS) are an important structural feature of the glymphatic system. PVS enlargement is associated with impaired glymphatic functionality and has been observed in both normative aging and neurodegenerative disorders; however, it is unclear how white matter PVS alterations affect neighboring cortical morphology in cognitively normal subjects. In the present study, we explore the relationship between PVS enlargement and cortical thickness across the normative lifespan. We found PVS enlargement preferentially influences cortical thickness of frontal regions, and this association is observed in children, adults and the elderly.
|
||
0324.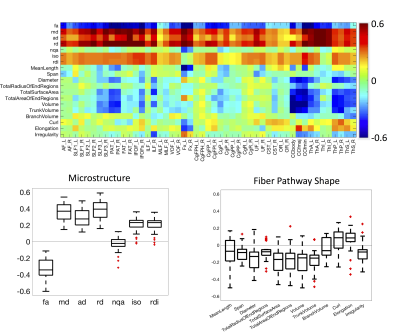 |
White matter shape and microstructure across the adult lifespan
Kurt G Schilling1, Fang-Cheng Yeh2, Leon Cai3, Colin Hansen3, Qi Yang3, Andrea T Shafer4, Susan Resnick4, Adam W Anderson3, and Bennett A Landman3
1Vanderbilt University Medical Center, Nashville, TN, United States, 2University of Pittsburgh Medical Center, Pittsburgh, PA, United States, 3Vanderbilt University, Nashville, TN, United States, 4National Institute on Aging, Baltimore, MD, United States
Here, we examine brain white matter diffusion magnetic resonance imaging data from a mixed longitudinal and cross-sectional dataset of 892 subjects and 1991 sessions of people aged 22.4-102.0 years from the Baltimore Longitudinal Study of Aging. Quantifying 7 microstructural features and, for the first time, 11 shape-based features across 49 white matter pathways, we document large age associations with white matter. Microstructure and shape measures are associated with age, although features of white matter shape do not show uniform trends across all pathways. Results from this study provide a comprehensive characterization of white matter pathways in the human brain.
|
||
 |
0325.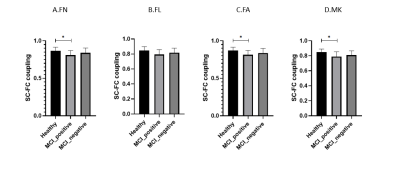 |
Alterations of Structural-Functional Coupling in Amyloid-Positive and Negative Mild Cognitive Impairment Patients
Hui Zhang1,2, Sai Kam Hui3, Peng Cao1, and Henry K.F. Mak1,2,4
1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 2Alzheimer's Disease Research Network, The University of Hong Kong, Hong Kong, Hong Kong, 3Department of Rehabilitation Science, The Hong Kong Polytechnic University, Hong Kong, Hong Kong, 4State Key Laboratory of Brain and Cognitive Sciences, The University of Hong Kong, Hong Kong, Hong Kong
To identify the structural and functional abnormalities in mild cognitive impairment (MCI) amyloid positive patients, combined resting state fMRI (rs-fMRI) and diffusion kurtosis imaging (DKI) were applied in this study. Graph theory metrics of subgroups were calculated and compared. In the results, MCI amyloid positive had impaired structural connectivity (SC) but not functional connectivity (FC) matrices and demonstrated significant SC-FC decoupling. We postulated that structural damage preceded functional reorganization. The pathological effects of fibrillar amyloid plaque toxicity occur in anatomical pathways, and functional reorganization might happen beyond the confines of structural pathways.
|
|
 |
0326.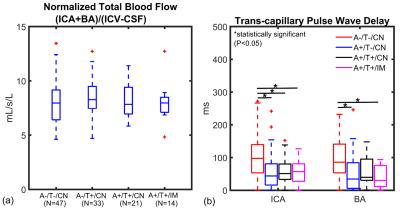 |
Associations in Alzheimer’s disease between Intracranial Vascular Metrics from 4D-Flow MRI and β-Amyloid and Tau PET
Leonardo A Rivera-Rivera1,2, Karly A Cody1, Tobey Betthauser1, Robert V Cadman1, Thomas Reher3, Howard A Rowley3, Cynthia M Carlsson1, Laura Eisenmenger3, Sterling C Johnson1, and Kevin M Johnson2,3
1Department of Medicine, University of Wisconsin-Madison, Madison, WI, United States, 2Department of Medical Physics, University of Wisconsin-Madison, Madison, WI, United States, 3Department of Radiology, University of Wisconsin-Madison, Madison, WI, United States
Cerebrovascular disease (CVD), has been linked with mild cognitive impairment and dementia stages of Alzheimer’s disease (AD); however, the question of whether CVD is associated with underlying AD pathophysiology remains unresolved. There remain many questions regarding CVD/AD pathophysiology interactions and whether related clinical AD dementia is enhanced by CVD. In this study, we investigated the relationship between cardiac and low frequency flow oscillations from 4D-Flow, white matter hyperintensities (WMHs) from T2 FLAIR MRI, and AD pathology assessed using β-amyloid (Aβ) and tau PET imaging data.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.