Combined Educational & Scientific Session
Fat & Metabolism
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 4 | 14:00 - 16:00 | Moderators: Pernilla Peterson & Mustafa Shadi Bashir |
 |
Chemical Shift MR Methods in Imaging Brown Adipose Tissue
Houchun Hu
This talk introduces the audience to MRI of brown adipose tissue (BAT). BAT studies in both animals and humans have progressed rapidly during the past decade. While positron emission and computed tomography (PET/CT) is likely to remain as a reference modality in BAT imaging, approaches using MRI have emerged as viable alternatives. MRI-based quantitative measurements of BAT fat content and metabolism are discussed. An outlook on research opportunities and future directions is provided.
|
|
 |
How to Quantify & Characterize Fat with MRI/MRS
Olof Dahlqvist Leinhard
This talk is about advanced body composition assessment methods using MRI and MRS. Approaches for automation and standardization across different scanner platforms will be discussed. Recent advancements in understanding how biomarkers for liver and muscle steatosis links to incidence and progression of metabolic disorders such as diabetes mellitus and coronary heart disease will be reviewed. By applying whole body MRI in large scale population studies, new phenotypes can be described connecting skewness in body fat distribution to specific comorbidity patterns. Further, it will be discussed how detailed volumetric description of muscles can advance our understanding of muscle degeneration and sarcopenia.
|
|
 |
Diagnostic Value of Quantifying Body Composition & Organ Fat
Shintaro Ichikawa
Non-alcoholic fatty liver disease (NAFLD) is a worldwide major public health problem. proton density fat fraction (PDFF) is a noninvasive quantitative evaluation method of hepatic steatosis. PDFF has excellent diagnostic value for assessment of liver fat accumulation and classification of histologic steatosis in patients with NAFLD. PDFF may be more sensitive than histological examination in detecting small changes in liver fat content. PDFF is a useful tool for the assessment of longitudinal changes of hepatic steatosis due to its noninvasiveness and high reproducibility. Recently, PDFF can also apply to other organs.
|
|
| Fat and Cancer
Vicky Goh
Normal adipose tissue is a dynamic structure with endocrine, metabolic, haematological and immune functions in addition to fat storage. Obesity (body mass index ≥30 kg/m2) has doubled in prevalence since the 1980s and is a risk to health. This lecture describes normal body fat distribution; the physiological changes in fat and muscle distribution with ageing; the prevalence of obesity globally and its impact on cancer risk and outcomes. Imaging techniques to assess body composition (fat-muscle mass) are compared and how such imaging can help the management of cancer patients is discussed.
|
| Concurrent 4 | 14:00 - 16:00 | Moderators: |
0751.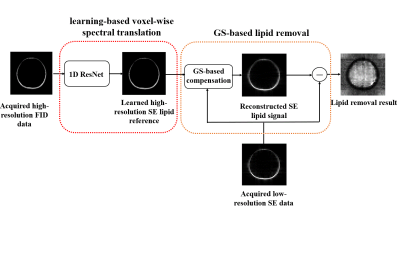 |
Removal of Subcutaneous Lipid Signals from Spin-Echo 1H-MRSI Brain Data Using an FID Reference and Machine Learning
Yunpeng Zhang1, Yibo Zhao2,3, Yudu Li2,3, Rong Guo2,3, Yao Li1, and Zhi-Pei Liang2,3
1School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China, 2Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 3Department of Electrical and Computer Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States
Spin-Echo (SE) MRSI can encode J-coupling information and is desirable for brain imaging applications. But it uses long TR, leading to long scan time and thus low resolution. Consequently, removal of subcutaneous lipid signals from low-resolution SE data is challenging. This paper presents a novel method to solve this problem. The proposed method uses a high-resolution FID reference and a neural network to transform it into SE signals, which are then used to construct a generalized-series model for lipid signal removal from the SE 1H-MRSI data. The proposed method has been tested using in vivo data, producing very encouraging results.
|
||
 |
0752.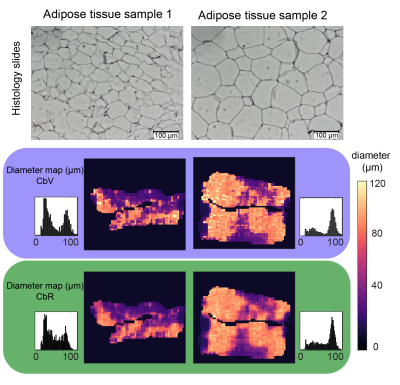 |
Lipid droplet size mapping in human adipose tissue using a clinical 3 T system
Dominik Weidlich1, Julius Honecker2, Christof Boehm1, Stefan Ruschke1, Daniela Junker1, Anh Van1, Marcus Makowski1, Christina Holzapfel3, Melina Claussnitzer4,5,6, Hans Hauner2,3, and Dimitrios C. Karampinos1
1Department of Diagnostic and Interventional Radiology, School of Medicine, Technical University of Munich, Munich, Germany, 2Else Kröner Fresenius Center for Nutritional Medicine, School of Life Sciences, Technical University of Munich, Munich, Germany, 3Institute for Nutritional Medicine, School of Medicine, Technical University of Munich, Munich, Germany, 4Broad Institute of MIT and Harvard, Cambridge, MA, United States, 5Beth Israel Deaconess Medical Center, Boston, MA, United States, 6Harvard Medical School, Harvard University, Boston, MA, United States
Despite its high relevance in metabolic research, the non-invasive measurement of adipocyte size remains an unmet need. DW-MRS has been previously applied to probe diffusion restriction effects in vivo to measure lipid droplets in animals up to diameters of 10 µm and in humans up to diameters of 50 µm. However, DW-MRS suffers from signal loss due to intravoxel-dephasing even when minimal motion is present. This work proposes a novel DW-MRI sequence and diffusion signal processing. In simulations and ex vivo adipose tissue measurements, the presented method results show agreement with ground truth and histology in measuring lipid droplet size.
|
|
 |
0753.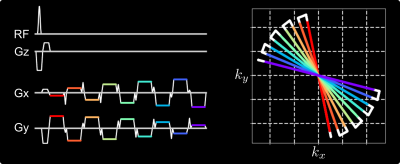 |
Free-Breathing Liver Fat and R2* Mapping: Multi-Echo Radial FLASH and Model-based Reconstruction (MERLOT)
Zhengguo Tan1,2, Sebastian Rosenzweig1,2, Xiaoqing Wang1,2, Nick Scholand1,2, H Christian M Holme1,2, Moritz Blumenthal1, and Martin Uecker1,2,3,4
1Institute for Diagnostic and Interventional Radiology, University Medical Center Göttingen, Göttingen, Germany, 2German Center for Cardiovascular Research (DZHK), Göttingen, Germany, 3Cluster of Excellence "Multiscale Bioimaging: from Molecular Machines to Networks of Excitable Cells" (MBExC), University of Göttingen, Göttingen, Germany, 4Campus Institute Data Science, University of Göttingen, Göttingen, Germany
To achieve free-breathing liver fat and $$$R_2^\star$$$ mapping, this work combines multi-echo radial FLASH with stack-of-stars volumetric acquisition and SSA-FARY to resolve respiratory motion. Moreover, regularized model-based reconstruction is implemented in BART to directly estimate quantitative parameter maps from acquired k-space data. Joint spatial and temporal regularization is used in this work. The proposed method is validated with NIST and water/fat phantoms. Furthermore, free-breathing liver studies show repeatability and good agreement between single-slice real-time and volumetric acquisition.
|
|
0754.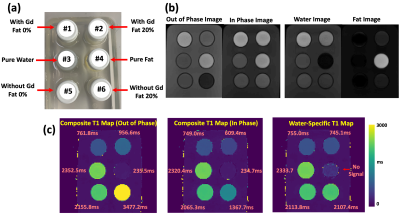 |
MP-Dixon-GRASP: Magnetization-Prepared Multiecho GRASP MRI for Free-Breathing Fat/Water-Separated 3D T1 Mapping
Li Feng1, Fang Liu2, Giorgios Soultanidis1, Chenyu Liu1, Thomas Benkert3, Sara Lewis1, Kai Tobias Block3,4, Zahi Fayad1, and Yang Yang1
1Biomedical Engineering and Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 2Department of Radiology, Massachusetts General Hospital, Harvard Medical School, Boston, MA, United States, 3MR Application Development, Siemens Healthcare GmbH, Erlangen, Germany, 4Center for Advanced Imaging Innovation and Research (CAI2R), New York University School of Medicine, New York, NY, United States
This study aims to develop a framework called MP-Dixon-GRASP (Magnetization-Prepared Dixon-based Golden-angle RAdial Sparse Parallel MRI) for rapid free-breathing fat/water-separated 3D T1 mapping of the liver. The technique combines inversion recovery-prepared multiecho stack-of-stars acquisition with subspace-based sparse image reconstruction. The performance of MP-Dixon-GRASP was evaluated in fat/water phantoms and in subjects with normal and elevated liver fat content. The results suggested that fat/water-separated T1 mapping is able to remove the influence of fat, which enables more accurate estimation of true T1 values in the liver. With fat/water-separated T1 estimation, MP-Dixon-GRASP could be potentially useful for imaging patients with fatty-liver diseases.
|
||
 |
0755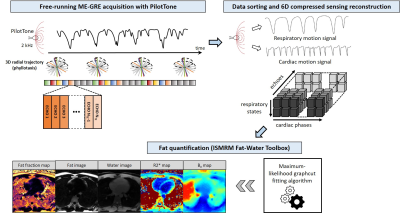 |
Whole-Heart Motion-Resolved Multi-Peak Fat-Fraction Mapping using Free-Running 3D Radial Multi-Echo GRE and Pilot Tone Video Permission Withheld
Adèle L. C. Mackowiak1, Christopher W. Roy1, Jérôme Yerly1,2, Lorenzo Di Sopra1, Mariana B. L. Falcaõ1, Mario Bacher1,3, Peter Speier3, Davide Piccini4,5, Matthias Stuber1,2, and Jessica A. M. Bastiaansen1
1Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 2CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 3Siemens Healthcare GmbH, Erlangen, Germany, 4Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, 5Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland A free-running multi-echo GRE approach was proposed for whole-heart fat quantification. Retrospective extraction of cardiac and respiratory motion states was achieved using integrated Pilot Tone navigation, enabling a free-breathing non-ECG-triggered acquisition. Following a motion-resolved compressed sensing based image reconstruction of the separate echoes, fat fraction, water fraction, R2* and B0 maps, as well as separated fat and water images, were calculated. Free-running acquisition parameters were optimized in a fat phantom. Volunteer experiments demonstrated the feasibility of motion-resolved free-running fat-fraction mapping technique in a 6-minute scan time. |
|
0756.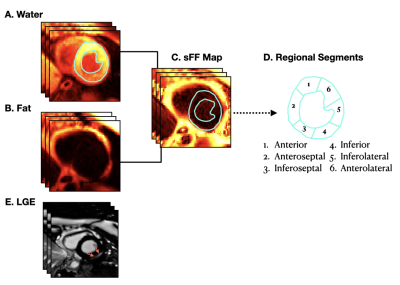 |
Left Ventricular Distribution of LGE and Intra-myocardial Fat in Boys with Duchenne Muscular Dystrophy and Healthy Controls at 3T
Nyasha G Maforo1,2, Ashley Prosper1, Pierangelo Renella1,3, Nancy Halnon4, Holden H Wu1,2,5, and Daniel B Ennis6,7,8
1Radiological Sciences, University of California, Los Angeles, Los Angeles, CA, United States, 2Physics and Biology in Medicine, University of California, Los Angeles, Los Angeles, CA, United States, 3Medicine (Pediatric Cardiology), Children's Hospital of Orange County, Orange, CA, United States, 4Pediatrics (Cardiology), University of California, Los Angeles, Los Angeles, CA, United States, 5Bioengineering, University of California, Los Angeles, Los Angeles, CA, United States, 6Radiology, Stanford University, Stanford, CA, United States, 7Cardiovascular Institute, Stanford University, Stanford, CA, United States, 8Maternal and Child Health Research Institute, Stanford University, Stanford, CA, United States
Duchenne muscular dystrophy (DMD) – a fatal X-linked genetic disorder – is characterized by progressive muscle weakness, pediatric onset cardiomyopathy, and ultimately heart failure. LGE imaging is the gold standard for detecting myocardial replacement fibrosis, but not fatty infiltration. We used chemical-shift based water-fat separation MRI techniques to investigate myocardial fibro-fatty infiltration and identify the onset of microstructural remodeling in boys with DMD. This study aimed to: 1) report and compare the left-ventricular (LV) intra-myocardial fat content in boys with DMD and healthy controls; and 2) determine whether fatty infiltration precedes the appearance of LGE in boys with DMD.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.