Digital Posters
Motion: Brain & Body
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 1 | 17:00 - 18:00 |
1371.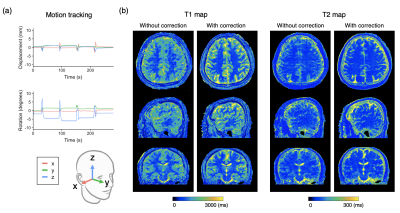 |
Prospective motion-corrected three-dimensional multiparametric mapping of the brain
Shohei Fujita1,2, Naoyuki Takei3, Akifumi Hagiwara1, Issei Fukunaga1, Dan Rettmann4, Suchandrima Banerjee5, Ken-Pin Hwang6, Shiori Amemiya2, Koji Kamagata1, Osamu Abe2, and Shigeki Aoki1
1Department of Radiology, Juntendo University, Tokyo, Japan, 2Department of Radiology, The University of Tokyo, Tokyo, Japan, 3MR Applications and Workflow, GE Healthcare, Tokyo, Japan, 4MR Applications and Workflow, GE Healthcare, Rochester, MN, United States, 5MR Applications and Workflow, GE Healthcare, Menlo Park, CA, United States, 6Department of Radiology, MD Anderson Cancer Center, Houston, TX, United States
A rigid real-time prospective motion-corrected multiparametric mapping technique was developed by inserting orthogonal spiral navigators into wait times of a multiparametric imaging technique, 3D-QALAS. The effects of motion correction on the quantitative estimates of a standardized phantom and in volunteers with various head motions were evaluated. Our results demonstrated that the proposed technique did not affect the accuracy of T1 and T2 quantification in vitro, and improved the repeatability and accuracy of T1 and T2 quantification with head motions during scans compared with those without motion correction. The proposed technique may provide robust three-dimensional multiparametric whole-brain mapping for head motions.
|
|||
1372.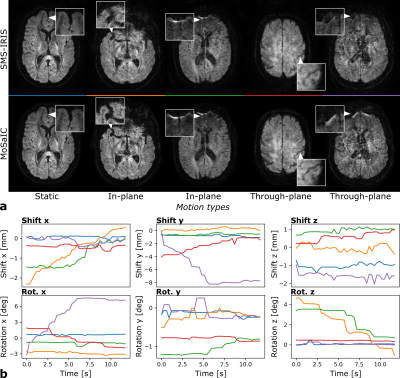 |
3D rigid motion correction for navigated interleaved simultaneous multi-slice DWI
Malte Riedel (né Steinhoff)1, Kawin Setsompop2,3,4, Alfred Mertins1, and Peter Börnert5,6
1Institute for Signal Processing, University of Lübeck, Lübeck, Germany, 2Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 3Department of Radiology, Harvard Medical School, Boston, MA, United States, 4Harvard‐MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 5Philips Research, Hamburg, Germany, 6Department of Radiology, C.J. Gorter Center for High-Field MRI, Leiden University Medical Center, Leiden, Netherlands
This work aims at improving the robustness of diffusion-weighted imaging (DWI) against 3D rigid head motion based on simultaneous multi-slice (SMS), interleaved echo-planar imaging (EPI) and image navigators. The proposed method utilizes low-resolution navigators to estimate diffusion phases as well as shot-wise 3D rigid motion through SMS-to-volume registration; providing high temporal resolution motion correction capability. The shot parameters are included into a full-volume reconstruction of the image data per diffusion direction. The method achieves submillimeter registration errors and improved image quality. In conclusion, the presented method enables retrospective 3D rigid motion correction for interleaved SMS DWI.
|
|||
1373.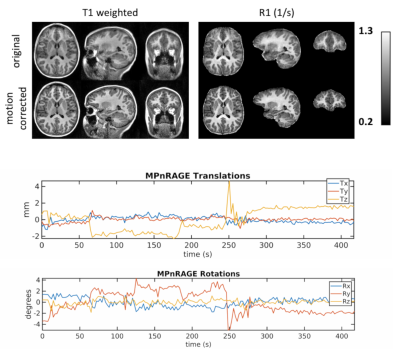 |
Cortical Mapping and T1-Relaxometry using Motion Corrected MPnRAGE: Test-Retest Reliability with and without Motion Correction
Steven Kecskemeti1, Abigail Freeman1, and Andrew L Alexander1
1University of Wisconsin-Madison, Madison, WI, United States
A test-retest study of FreeSurfer cortical thickness, surface area, and volume, as well as cortical R1 relaxometry, was performed on pediatric subjects scanned without sedation using SNARE-MPnRAGE. Reliability was assessed with coefficients of variation (CoVs) and intraclass correlation coefficients (ICCs). When SNARE motion correction was used all parameters had statistically significant improvements and high reliability. For the mean (thickness/surface area/volume/R1) across the regions of FreeSurfer’s DK Atlas, the mean CoVs (% x100) were (1.2/1.6/1.9/0.9) and the mean ICCs were (0.88/0.96/0.94/0.83). When assessed on a per-vertex basis, the CoVs and ICCs for thickness/R1 had mean values of (2.9/1.9) and (0.82/0.68).
|
|||
1374.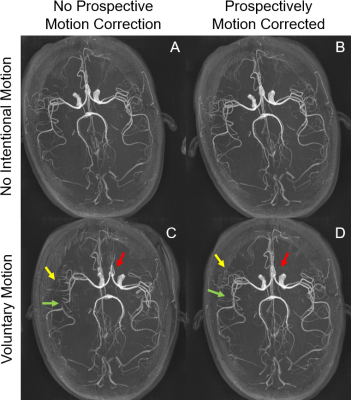 |
Prospective Motion Corrected Time-of-flight MR Angiography at 3T
Xiaoke Wang1, Edward Herskovits1, and Thomas Ernst1
1Diagnostic Radiology, University of Maryland-Baltimore, Baltimore, MD, United States
MRA at 3T is sensitive to patient motion which sometimes occur due to severe illness. This work investigated the effect of optic prospective motion correction (PMC) on the MRA at 3T. MRA with optic PMC was tested in phantom and on a healthy volunteer and compared with MRA without PMC. The use of PMC successfully reversed the slab misalignment, artifactual stenosis, and discontinuous major arteries caused by the intentional motion by the volunteer, and enhanced the distal vessels. This study demonstrated the potential of optic PMC in improving the quality of MRA on patients with difficulty holding still.
|
|||
1375.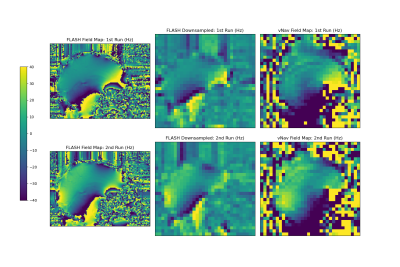 |
Dual-echo volumetric navigators for field mapping and shim correction in MR neuroimaging
Alan Chu1, Yulin Chang2, André J. W. van der Kouwe3, and M. Dylan Tisdall1
1Radiology, Perelman School of Medicine, University of Pennsylvania, Philadelphia, PA, United States, 2Siemens Medical Solutions USA, Inc., Malvern, PA, United States, 3Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States
We demonstrate the feasibility and validity of field mapping using a dual-echo vNav based on a fly-back EPI readout. A human subject was scanned using the proposed method and a standard FLASH acquisition for comparison. The proposed method does not require additional reconstruction or acquisition complexity and is easily computed on typical MR scanners to support real-time motion and shim correction.
|
|||
1376.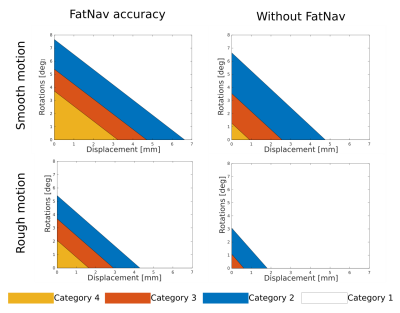 |
Comprehensive Analysis of FatNav Motion Parameters Estimation Accuracy in 3D Brain Images Acquired at 3T
Elisa Marchetto1,2, Kevin Murphy1,3, and Daniel Gallichan1,2
1Cardiff University Brain Research Imaging Centre (CUBRIC), Cardiff University, Cardiff, United Kingdom, 2School of Engineering, Cardiff University, Cardiff, United Kingdom, 3School of Physics, Cardiff University, Cardiff, United Kingdom
FatNav motion-parameter estimation relies on GRAPPA reconstruction of the highly accelerated navigator fat-volumes, which might be compromised by strong changes in the head position. Data from three MPRAGE brain images have been used to find the motion corresponding to four image quality boundaries and assess motion tolerance when FatNavs are used. Results suggests that FatNavs can compensate for a large range of motion artifacts compared to when no motion correction is applied. Better correction is expected if GRAPPA weights are updated throughout the entire duration of the scan.
|
|||
1377.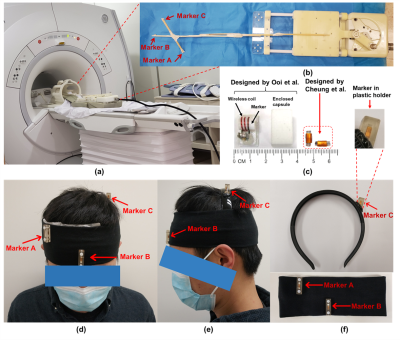 |
Head Motion Tracking in MRI Using Novel Tiny Wireless Tracking Markers and Projection Signals
Liyuan LIANG1, Chim-Lee Cheung2, Ge Fang2, Justin Di-Lang Ho2, Chun-Jung Juan3,4,5, Hsiao-Wen Chung6, Ka-Wai Kwok2, and Hing-Chiu Chang1
1Department of Diagnostic Radiology, The University of Hong Kong, Hong Kong, Hong Kong, 2Department of Mechanical Engineering, The University of Hong Kong, Hong Kong, Hong Kong, 3Department of Medical Imaging, China Medical University Hsinchu Hospital, Hsinchu, Taiwan, 4Department of Radiology, School of Medicine, College of Medicine, China Medical University, Taichung, Taiwan, 5Department of Medical Imaging, China Medical University Hospital, Taichung, Taiwan, 6Department of Electrical Engineering, National Taiwan University, Taipei, Taiwan
Head motion is a significant problem for the challenging populations, and the wireless tracking coils has previously been proposed to enable prospective motion correction in MRI. In this study, we evaluated the tracking performance of a novel tiny wireless tracking marker by using a linear motion phantom, and tested the feasibility in omnidirectional 3D head motion tracking using three tiny wireless tracking markers. Both phantom and in-vivo results suggest that the novel tiny wireless tracking markers can provide good fidelity in 3D position tracking, with improved subject comfort and better flexibility in fixation of markers.
|
|||
1378.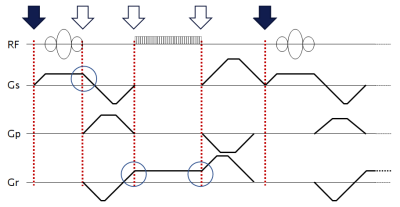 |
Real-time prospective motion correction of arbitrary MR pulse sequences with XPACE-Pulseq
Maxim Zaitsev1, Michael Woletz1, and Martin Tik1
1High Field MR Center, Center for Medical Physics and Biomedical Engineering, Medical University of Vienna, Vienna, Austria
Although it is theoretically possible to prospectively correct for deteriorative effects of known rigid body motions in any arbitrary MRI pulse sequence, no practical open implementation was reported to date. In this work we extend Puseq, an open-source pulse sequence development environment with a prospective motion correction capability based on external tracking. Real-time motion information in six degrees of freedom is received from an optical motion tracking system by libXPACE (a generic library for eXternal Prospective Acquisition CorrEction). The framework is capable of both motion correction and motion artifact simulation of arbitrary pulse sequences saved in Pulseq format.
|
|||
1379.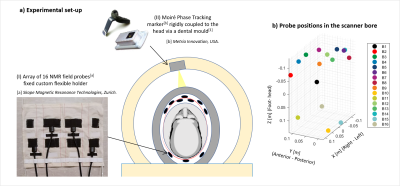 |
Measuring extracranial magnetic field changes due to head motion during multi-slice EPI acquisition
Laura Bortolotti1 and Richard Bowtell1
1Sir Peter Mansfield Imaging Centre, University of Nottingham, Nottingham, United Kingdom
A novel approach to motion tracking has been successfully tested during a EPI scan in a 7T scanner. A set of NMR field probes, placed in between the standard head transmit and receiver coils, was used to measure the extra-cranial field changes due to head movement every 150 ms during quiet periods of the EPI sequence. These measurements were used in conjunction with a regression method to predict head motion parameters. This represents a step forward in integrating a marker-less motion correction technique with standard imaging.
|
|||
1380. |
Structure Light based Optical MOtion Tracking system (SLOMO) for Contact-free respiratory Motion Tracking from Neck in MR Imaging
Chunyao Wang1, Zhensen Chen1, Yishi Wang2, and Huijun Chen1
1Center for Biomedical Imaging Research, School of Medicine, Tsinghua University, Beijing, China, 2Philips Healthcare, Beijing, China
This study proposed a parallel line Structure Light based Optical Motion Tracking system (SLOMO) and verified its feasibility in respiratory detection and motion correction in MR liver imaging.
|
|||
 |
1381.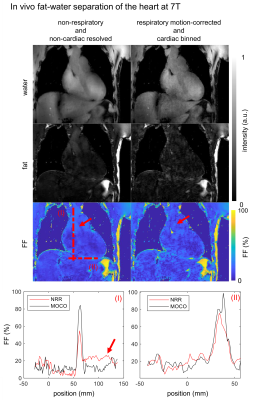 |
Respiratory resolved and corrected 3D $$$\Delta\text{B0}$$$ mapping and fat-water imaging at 7 Tesla
Sebastian Dietrich1, Johannes Mayer1, Christoph Stephan Aigner1, Christoph Kolbitsch1, Jeanette Schulz-Menger2,3,4, Tobias Schaeffter1,5, and Sebastian Schmitter1,6
1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berin, Germany, 2Charité Medical Faculty University Medicine, Berlin, Germany, 3DZHK partner site Berlin, Working Group on Cardiovascular Magnetic Resonance, Experimental and Clinical Research Center (ECRC), Berlin, Germany, 4Department of Cardiology and Nephrology, HELIOS Klinikum Berlin Buch, Berlin, Germany, 5Department of Medical Engineering, Technische Universität Berlin, Berlin, Germany, 6University of Minnesota, Center for Magnetic Resonance Research, Minneapolis, MN, United States
This work demonstrates 3D respiratory motion-corrected and cardiac resolved fat-water separation in the human body at 7T. Accurate fat fraction quantification using bipolar readout gradients was validated in a phantom. The impact of motion compensation on the underlying motion resolved $$$\Delta\text{B0}$$$ maps is investigated and estimated. In total the 3D fat-water separation and fat fraction quantification is successfully demonstrated in 10 healthy volunteers at 7T with a large BMI range from $$$19$$$ to $$$34 \text{kg/m}²$$$.
|
||
1382.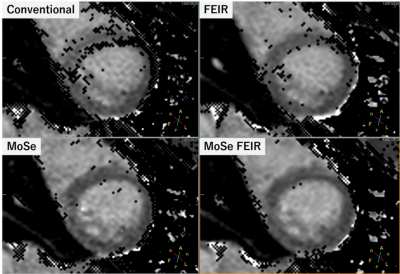 |
Minimizing motion artifacts in myocardial quantitative mapping by combined use of motion-sensitive CINE imaging and FEIR
Takumi Ogawa1, Michinobu Nagao2, Masami Yoneyama3, Yasutomo Katsumata3, Yasuhiro Goto1, Isao Shiina1, Yutaka Hamatani1, Kazuo Kodaira1, Mamoru Takeyama1, Isao Tanaka1, and Shuji Sakai2
1Department of Radiological Services, Women's Medical University Hospital, tokyo, Japan, 2Department of Diagnostic imaging & Nuclear Medicine, Women's Medical University Hospital, tokyo, Japan, 3Philips Japan, tokyo, Japan
Myocardial mapping such as T1 and T2 is widely used clinically for evaluating the properties of myocardium. We evaluated the feasibility of the combined use of Motion-Sensitive (MoSe) CINE imaging for determining accurate TD setting and fast elastic image registration (FEIR), which is a registration-based nonrigid motion correction for minimizing the influence of cardiac motion-related artifacts.
|
|||
1383.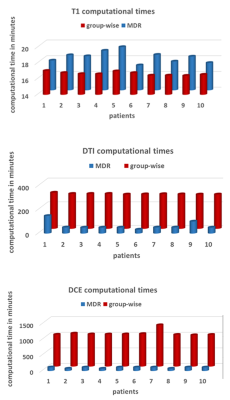 |
Model-based motion correction outperforms a model-free method in quantitative renal MRI
Fotios Tagkalakis1, Kanishka Sharma2, Irvin Teh1, Bashair al-Hummiany1, David Shelley1, Margaret Saysell3, Julie Bailey3, Kelly Wroe3, Cherry Coupland3, Michael Mansfield3, and Steven Sourbron2
1University of Leeds, Leeds, United Kingdom, 2University of Sheffield, Sheffield, United Kingdom, 3Leeds Teaching Hospitals NHS Trust, St James's Hospital, United Kingdom
Current approaches for motion correction of quantitative MRI include model-driven registration (MDR) and model-free registration (MFR). This study compared MDR against a state-of-the-art groupwise MFR (GMFR) on T1-mapping, DTI and DCE from 10 patients with diabetic kidney disease. The results demonstrate the benefits of MDR in the context of quantitative imaging: MDR scores better on most quality and error metrics (up to 20% improvement) and offers a substantial gain in computation times (up to 17hrs per slice). Furthermore, there is potential for translation to other applications.
|
|||
1384.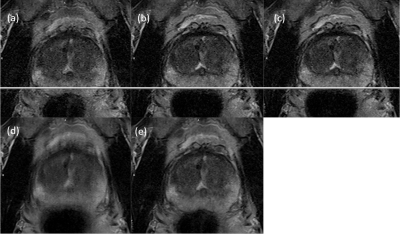 |
Motion-robust T2-weighted TSE imaging in the prostate by performing non-rigid registration between averages
Katja Bogner1, Elisabeth Weiland2, Thomas Benkert2, and Karl Engelhard1
1Institute of Radiology, Martha-Maria Hospital, Nuremberg, Germany, 2MR Application Predevelopment, Siemens Healthcare GmbH, Erlangen, Germany
The major reason for artifacts in T2-weighted prostate imaging is motion-induced blurring caused by slight displacements between averages. Here, a non-rigid elastic registration is proposed to properly align the images before averaging. The method is evaluated in 12 patients and is shown to improve image quality in 71% of all investigated cases. Identification of pathology was improved in 42%. While only 58% of the conventional images achieved good or excellent image quality, this was the case for 88% when applying motion correction. As demonstrated, non-rigid registration results in clearly reduced motion artifacts and improves image quality and diagnostic confidence.
|
|||
1385.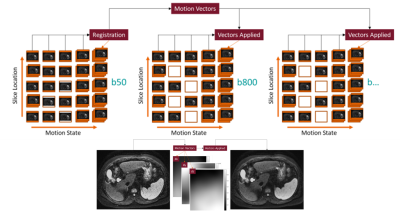 |
Motion Correction of Abdominal Diffusion-Weighted MRI Using Internal Motion Vectors
Michael Bush1, Thomas Vahle2, Uday Krishnamurthy1, Thomas Benkert2, Xiaodong Zhong1, Bradley Bolster1, Paul Kennedy3, Octavia Bane3, Bachir Taouli3, and Vibhas Deshpande1
1Siemens Medical Solutions USA, Inc., Malvern, PA, United States, 2Siemens Healthcare GmbH, Erlangen, Germany, 3The Department of Radiology and Biomedical Engineering and Imaging Institute, Icahn School of Medicine at Mt. Sinai, New York, NY, United States
Respiratory motion is a significant problem in MRI and is further amplified in abdominal imaging. Traditional methods to correct for motion in abdominal DWI significantly increase overall scan time. In this work, motion vectors derived from non-rigid registration of low b-value diffusion volumes are used to correct for motion in higher b-values, where low signal to noise and contrast make intra and inter b-value registration challenging. Initial results suggest the proposed method can produce images similar in quality to respiratory gating, while maintaining reduced acquisition times.
|
|||
1386.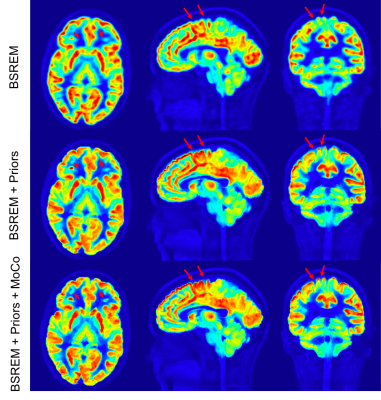 |
High Resolution PET/MR Imaging Using Anatomical Priors & Motion Correction
Mehdi Khalighi1, Timothy Deller2, Floris Jansen2, Mackenzie Carlson3, Tyler Toueg4, Steven Tai Lai1, Dawn Holley1, Kim Halbert1, Elizabeth Mormino4, Jong Yoon1, Greg Zaharchuk1, and Michael Zeineh1
1Radiology, Stanford University, Stanford, CA, United States, 2Engineering Dept., GE Healthcare, Waukesha, WI, United States, 3Bioengineering, Stanford University, Stanford, CA, United States, 4Neurology, Stanford University, Stanford, CA, United States
Using the anatomical priors in PET image reconstruction by exploiting the correlation between similar voxels in addition to the correlation between neighboring voxels to control noise, will improve the image quality and resolution. However, motion can degrade the outcome if they anatomical priors are not perfectly registered with PET images. Here we have combined PET image reconstruction with anatomical priors and rigid motion correction for PET/MR brain images to address this. The results show improved image resolution in addition to higher signal-to-noise ratio.
|
|||
1387. |
Ultra-wide-band radar for respiratory motion correction of T1 mapping in the liver
Tom Neumann1, Juliane Ludwig1, Kirsten M. Kerkering1, Frank Seifert1, and Christoph Kolbitsch1
1Physikalisch-Technische Bundesanstalt (PTB), Braunschweig and Berlin, Germany
In this work we introduce a motion correction approach based on a calibrated ultra-wide-band radar signal acquired simultaneously to the MR scan. Our method is contactless and entirely independent from scanner setups or applied imaging sequences which makes it a viable alternative to established motion correction approaches, like navigator sequences or breathholds. We could demonstrate in phantom and in-vivo scans that the proposed motion-correction approach strongly improves T1 quantification.
|
|||
1388.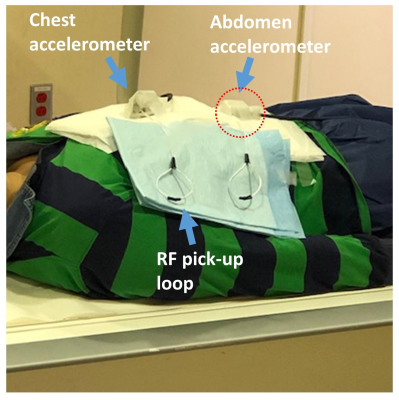 |
Detecting Respiratory Motion Using Accelerometer Sensors: Preliminary Insight
Eddy Solomon1,2, Syed Saad Siddiq1,2, Daniel K Sodickson1,2, Hersh Chandarana1,2, and Leeor Alon1,2
1Radiology, New York University School of Medicine, New York, NY, United States, 2New York University Grossman School of Medicine, New York, NY, United States
MRI scans are often under continues involuntary motion which weakens their reliability and diagnostic utility for examining the chest and abdomen. Acquisitions using traditional external sensors (e.g. respiratory belt) and self-gated techniques tend to be highly sensitive to patient position and setup, and on MR sequence parameters. Here, we demonstrate the use of accelerometer sensors for detecting respiratory signals. We show how the use of this simple sensor, with its relatively small dimensions, high sampling rate capability, and low cost, can produce motion corrected-images under free-breathing conditions.
|
|||
1389.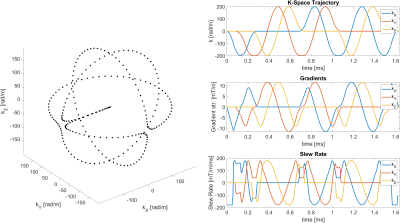 |
Detection of Head Motion using Navigators and a Linear Perturbation Model
Thomas Ulrich1 and Klaas Paul Pruessmann1
1Institute for Biomedical Engineering, ETH Zurich and University of Zurich, Zurich, Switzerland
We propose a novel algorithm for motion estimation with k-space navigators. The algorithm operates on the complex k-space navigator signal. It uses a linear signal model, which describes how the navigator signal changes under rotation and translation of the imaged object. Rotation and translation are estimated simultaneously by means of linear least-squares parameter fitting using the linear model. We show that the algorithm achieves high accuracy and precision through a phantom experiment with a motionless phantom. Furthermore, we show the algorithms's applicability in-vivo during a volunteer experiment with and without intentional head motion.
|
|||
1390.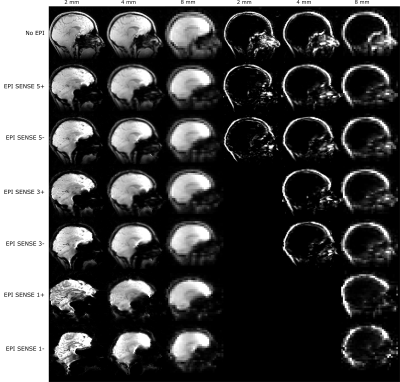 |
Effects of geometric distortions on navigator accuracy for motion corrected brain imaging at 7T
Mads Andersen1 and Vincent Oltman Boer2
1Philips Healthcare, Copenhagen, Denmark, 2Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital Hvidovre, Hvidovre, Denmark
High resolution structural brain imaging at 7T can benefit from motion correction with volumetric navigators. Echo-planar-imaging (EPI) can reduce navigator durations and therefore allow for higher resolution navigators which are tempting to use to increase accuracy of the movement parameters. However, the severe B0 inhomogeneities at 7T lead to geometric distortions that change with head position and therefore lower navigator accuracy. We investigated the navigator accuracy for water and fat navigators of different resolutions and EPI readout durations. We found that the realignment parameter error grows with the amount of motion, voxel size, and EPI readout duration.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.