Digital Posters
Artifacts & Corrections Related to the System & Sample
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 1 | 13:00 - 14:00 |
3538.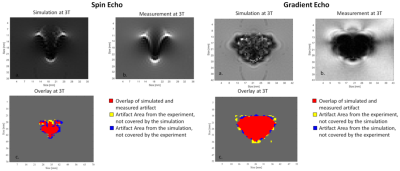 |
Development and evaluation of a numerical simulation approach to predict metal artifacts from passive implants in MRI
Tobias Spronk1,2,3, Oliver Kraff1, Gregor Schaefers3,4, and Harald H Quick1,2
1Erwin L. Hahn Institute for MR Imaging, University of Duisburg-Essen, Essen, Germany, 2High-Field and Hybrid MR Imaging, University Hospital Essen, Essen, Germany, 3MRI-STaR Magnetic Resonance Institute for Safety, Technology and Research GmbH, Gelsenkirchen, Germany, 4MR:comp GmbH, Testing Services for MR Safety & Compatibility, Gelsenkirchen, Germany
This study presents a numerical approach to simulate artifacts of metallic implants in the MR environment, which can be applied to improve the MR image artifacts testing procedure for medical implants according to ASTM F2119. The numerical approach is validated by comparing simulations and measurements of two metallic test objects made of titanium and stainless steel at three different field strengths (1.5 T, 3 T, and 7 T).
|
|||
3539.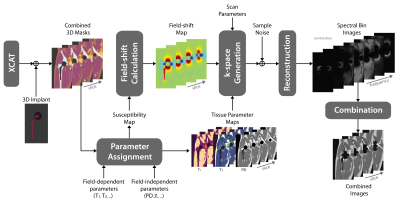 |
Realistic Simulation of MRI Metal Artifact and Field Strength Dependence
Kübra Keskin1, Brian Hargreaves2,3,4, and Krishna S. Nayak1,5
1Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States, 2Radiology, Stanford University, Stanford, CA, United States, 3Electrical Engineering, Stanford University, Stanford, CA, United States, 4Bioengineering, Stanford University, Stanford, CA, United States, 5Biomedical Engineering, University of Southern California, Los Angeles, CA, United States
MRI near metallic implants suffers from severe artifacts due to magnetic susceptibility, and these artifacts scale with the B0 field strength. Techniques like “view angle tilting” and “multi spectral imaging” partially mitigate these distortions and are widely used at 1.5T and 3T. Here, we provide a pipeline for realistic simulation of MRI metal artifacts and noise using anatomic digital phantoms and susceptibility calculations. For one example application, total hip arthroplasty, we demonstrate the impact of field strength (0.2T, 0.55T, 1.5T, 3T), and MSI parameters such as receiver bandwidth, RF bandwidth, and number of spectral bins.
|
|||
3540.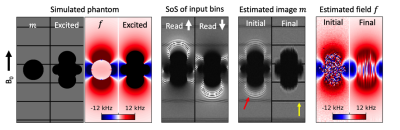 |
Joint estimation of image content and field inhomogeneity for artifact correction near metallic implants
Alexander R Toews1,2, Daehyun Yoon1, and Brian A Hargreaves1,2,3
1Radiology, Stanford University, Stanford, CA, United States, 2Electrical Engineering, Stanford University, Stanford, CA, United States, 3Bioengineering, Stanford University, Stanford, CA, United States
Multispectral imaging sequences mitigate geometric distortion near metallic implants at the expense of readout blur and residual intensity artifacts. To address these limitations, a method for jointly estimating the image content and field inhomogeneity near metal is presented. A dual polarity readout acquisition is used to provide sufficient conditioning for the estimation problem. The method is studied in simulation as well as in a physical phantom consisting of a shoulder implant embedded in a resolution grid. Results indicate that the method can greatly reduce readout blur and in some cases eliminate residual intensity artifacts.
|
|||
3541.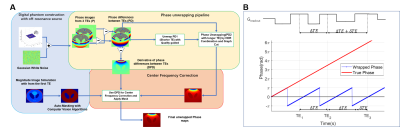 |
Accurate mUlti-echo phase image wiTh uneven echO spacing and Ultra-High Dynamic Range (AUTO-HDR)
Yuheng Huang1,2, Xinheng Zhang1,2, Serry Fradad1, Lu Meng1, Ghazal Yoosefian1, Linda Azab1, Xinqi Li3, Alan Kwan4, Rohan Dharmakumar1, Hui Han1, and Hsin-Jung Yang1
1Biomedical Imaging Research Institute, Cedars Sinai Medical Center, Los Angeles, CA, United States, 2Bioengineering, UCLA, Los Angeles, CA, United States, 3Columbia University, New York, NY, United States, 4Cardiology, Cedars Sinai Medical Center, Los Angeles, CA, United States
A strong off-resonance field around metallic implants induces unreliable phase maps and limits the application of phase-based MRI techniques. State-of-the-art MR phase mapping and unwrapping techniques perform poorly under the influence of metallic implants because of their limited dynamic range and susceptibility to noise. In this study, we developed a fully automatic phase mapping technique based on a multi-echo GRE sequence with unevenly spaced echoes and a high-dynamic-range phase reconstruction algorithm. We tested the proposed method with numerical simulations and human subjects with metallic implants.
|
|||
3542.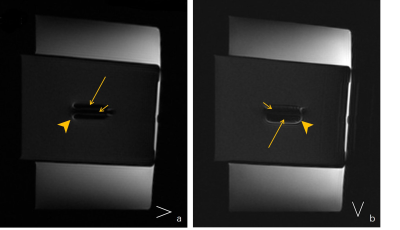 |
Directional effect of frequency-encoding gradient on T2WI-STIR imaging: a phantom study to evaluate the metal artifact
Xu Lulu1, Shen Yong2, Dou Weiqiang3, and Qi Liang1
1Radiology, Jiangsu Province Hospital, Nanjing, China, 2GE Healthcare, MR Enhanced Application China, Beijing, China, 3GE Healthcare, MR Research China, Beijing, China
This study aimed to determine the optimal direction of the frequency-encoding gradient when acquiring T2WI-STIR images with titanium alloy. To investigate this, we made in vitro MRI experiment. The water-pork phantom with ten different-length titanium alloys was constructed, and the short- and long-axis ratios calculated in different frequency-encoding directions were compared with each other, respectively. We found that when the frequency-encoding axis was perpendicular to the screw, the metallic artifact was smaller in whatever long axis or short axis. This result may improve observing more anatomical structures around the embedded metal.
|
|||
3543.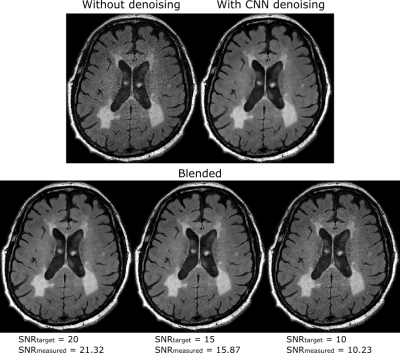 |
Image blending method to produce consistent SNR in images denoised using a convolutional neural network
Anuj Sharma1 and Andrew J Wheaton1
1Magnetic Resonance, Canon Medical Research USA, Inc., Mayfield Village, OH, United States
Deep learning based denoising methods can significantly reduce image noise at the cost of producing unnaturally smooth images. Typically, natural-looking images are produced by blending a fraction of the acquired noisy image with the denoised image. The blending ratio is set based on visual inspection. This approach impedes workflow and is prone to inter-operator variability. We propose a method to analytically calculate the blending ratio based on a desired SNR value. The proposed method is demonstrated to produce natural-looking denoised images with consistent SNR across head, spine and knee applications.
|
|||
3544.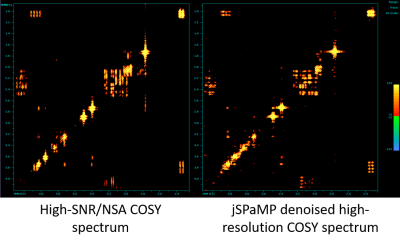 |
Denoising MR 2D COSY spectra using a joint sparse parametric Matching Pursuit (jSPaMP)
Boris Mailhe1, Zahra Hosseini2, Bing Ji3, Hui Mao3, and Mariappan S. Nadar1
1Digital Technology and Innovation, Siemens Healthineers, Princeton, NJ, United States, 2MR R&D Collaboration, Siemens Medical Solutions USA Inc., Atlanta, GA, United States, 3Department of Radiology and Imaging Sciences, Emory University, Atlanta, GA, United States
This work introduces a new approach to denoise 2D COSY data that typically are collected with low number of signal averages. Our method of a joint sparse parametric Matching Pursuit uses high-resolution parametric models to model the behavior of 2D COSY data in the F2 dimension and add nonparametric joint sparse constraints to regularize the peak shape in the F1 dimension. The effect and performance of denoising were demonstrated using a phantom.
|
|||
3545.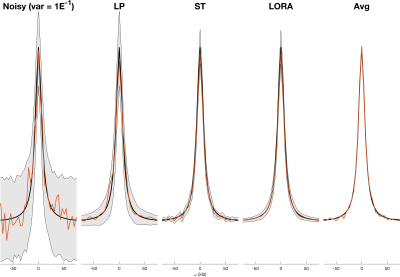 |
Characterising the variance and reproducibility of low rank denoising methods for spectroscopic data
William T Clarke1 and Mark Chiew1
1Wellcome Centre for Integrative Neuroimaging, NDCN, University of Oxford, Oxford, United Kingdom
Low rank denoising methods can reduce the noise present in spectroscopic data, a typically signal-to-noise limited technique. Low rank denoising is a data driven technique, typically exploiting the linear predictability or spatio-temporal separability of the data. Despite high levels of apparent denoising it is not clear whether denoising decreases the final uncertainty in the measurement, as the denoised data can be biased. In this work we assess the uncertainty reduction using Monte Carlo simulation and by measuring reproducibility of noisy and denoised 1H MRSI. We find low rank denoising reduces uncertainty but by much less than is apparent.
|
|||
3546.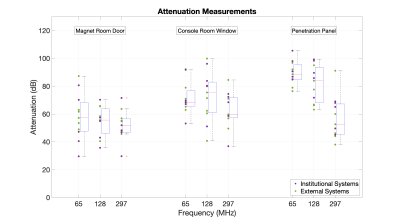 |
A Survey of Faraday Cage Attenuation Measurements of Clinical MRI Systems
Francesco Padormo1, Joe Martin1, Jane Ansell1, Elizabeth Gabriel1, Laurence H. Jackson1, Caitlin O'Brien1, Simon Shah1, David Price1, and Geoff Charles-Edwards1
1Guy's & St Thomas' NHS Foundation Trust, London, United Kingdom
Manufacturers typically specify new MRI systems to have a minimum Faraday Cage (FC) attenuation of 90-100dB at the resonant frequency. Anecdotal observations have suggested that systems can operate successfully with FC attenuation levels below this specification. We present a survey of attenuation measurements across eleven systems in order to gain insight into realistic values on systems in routine clinical use.
|
|||
3547. |
Usefulness of Deep Learning Based Denoising Method for Compressed Sensing in Pituitary MRI
Takeshi Nakaura1, Hiroyuki Uetani1, Kousuke Morita1, Kentaro Haraoka2, Akira Sasao1, Masahiro Hatemura1, and Toshinori Hirai1
1Diagnostic Radiology, Kumamoto University, Kumamoto, Japan, 2Cannon Medical Systems Japan, Tochigi, Japan
We evaluated image quality of hybrid type deep learning reconstruction (hybrid-DLR) with wavelet based denoising method in T2-weighted images (T2WI) of the pituitary with various denoising level (1-5). There was a progressive increase in SNR with hybrid-DLR with increase of the denoising level. On the other hand, the SNR of conventional wavelet-based method was not increased at high denoising levels (4-5). All qualitative scores of hybrid-DLR in any denoising levels are higher than that of wavelet based denoising method, and the difference became more noticeable at higher denoising levels.
|
|||
3548.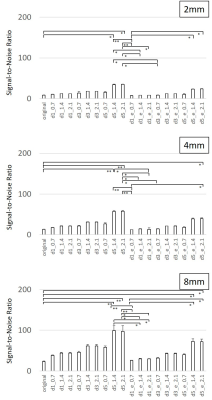 |
Evaluation of noise reduction performance using deep learning reconstruction: A phantom study
Hitoshi Kubo1, Yuya Abe2, Tomoya Yokokawa2, Seira Yokoyama2, and Koji Hoshi2
1Fukushima Medical University, Fukushima, Japan, 2Hoshi General Hospital, Koriyama, Japan
We aim to assess fundamental noise reduction performance using deep learning reconstruction with a phantom at a 1.5 T MR scanner. In this study, the relationship among parameters for noise reduction, signal-to-noise ratio, image quality, and spatial resolution of images was evaluated. SNRs were increased higher significantly by DLR in all SNR ranges. Increasing ratio of SNR was varied by means of parameter settings. Combination of the DLR parameters affected varies to SNR, SSIM, and spatial resolution of the images. We should exercise caution to select DLR parameters when this technique applies to clinical images.
|
|||
| 3549. | Suppressing the ballistocardiography artifacts on EEG collected inside MRI using the dynamic modeling on heartbeats
Hsin-Ju Lee1,2, Hsiang-Yu Yu3,4,5, Cheng-Chia Lee4,5,6, Chien-Chen Chou3,4, Chien Chen3,4, Wen-Jui Kuo5,7, and Fa-Hsuan Lin1,2,8
1Physical Sciences Platform, Sunnybrook Research Institute, Toronto, ON, Canada, 2Department of Medical Biophysics, University of Toronto, Toronto, ON, Canada, 3Department of Epilepsy, Neurological Institute, Taipei Veterans General Hospital, Taipei, Taiwan, 4School of Medicine, National Yang-Ming University, Taipei, Taiwan, 5Brain Research Center, National Yang-Ming University, Taipei, Taiwan, 6Department of Neurosurgery, Neurological Institute, Taipei Veterans General Hospital, Taipei, Taiwan, 7Institute of Neuroscience, National Yang-Ming University, Taipei, Taiwan, 8Department of Neuroscience and Biomedical Engineering, Aalto University, Espoo, Finland
We developed the dynamic modeling of heartbeats (DMH) method to suppress the ballistocardiography (BCG) artifacts on the electroencephalography (EEG) data collected inside MRI. DMH estimates the instantaneous EEG signals at specific phases in the cardiac cycle by combining EEG signals at those phases in other cardiac cycles showing similar dynamic features. Using both simulations and empirical data at 3T, we demonstrated that the DMH approach can suppress the BCG artifacts more efficiently than Optimal Basis Set (OBS) method in both epilepsy and steady-state visual evoked potential data.
|
|||
3550.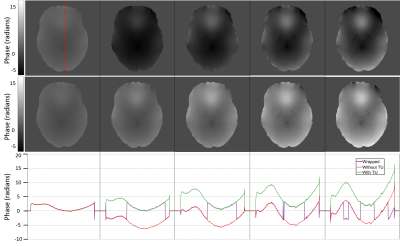 |
Robust spatial and temporal unwrapping for accurate quantitative susceptibility mapping
Alex Ensworth1, Véronique Fortier1,2, Jorge Campos Pazmino1, and Ives R Levesque1,2,3,4
1Medical Physics Unit, McGill University, Montreal, QC, Canada, 2Biomedical Engineering, McGill University, Montreal, QC, Canada, 3Research Institute of the McGill University Health Centre, Montreal, QC, Canada, 4Gerald Bronfman Department of Oncology, McGill University, Montreal, QC, Canada
Phase data for quantitative susceptibility mapping must be unwrapped to remove ambiguities due to limitations in the collected data. This work demonstrates the advantages of quality-guided region-growing over conventionally used methods and introduces a general method to correct for temporal discontinuities introduced by path-based spatial unwrapping methods. The unwrapping approaches were evaluated in 11 in vivo datasets presenting a variety of phase profiles.
|
|||
3551.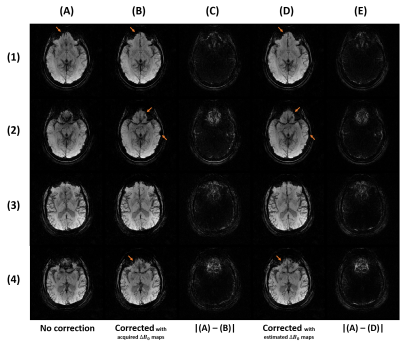 |
Off-resonance correction of non-cartesian SWI using internal field map estimation
Guillaume Daval-Frérot1,2,3, Aurélien Massire1, Mathilde Ripart2, Boris Mailhe4, Mariappan Nadar4, Alexandre Vignaud2, and Philippe Ciuciu2,3
1Siemens Healthcare SAS, Saint-Denis, France, 2CEA, NeuroSpin, CNRS, Université Paris-Saclay, Gif-sur-Yvette, France, 3Inria, Parietal, Palaiseau, France, 4Digital Technology and Innovation, Siemens Healthineers, Princeton, NJ, United States
Patient-induced inhomogeneities in the magnetic field cause distortions and blurring during acquisitions with long echo times, as in susceptibility-weighted imaging. Most correction methods require collecting an additional $$$\Delta{B_0}$$$ field map. To avoid that, we propose a method to approximate this field map using the single echo acquisition only. The main component of the observed phase is linearly related to $$$\Delta{B_0}$$$ and TE, and the relative impact of non-$$$\Delta{B_0}$$$ terms becomes insignificant with TE>20ms at 3T. The estimated 3D field maps, produced at 0.6 mm isotropic under 3 minutes, provide corrections equivalent to acquired ones.
|
|||
3552.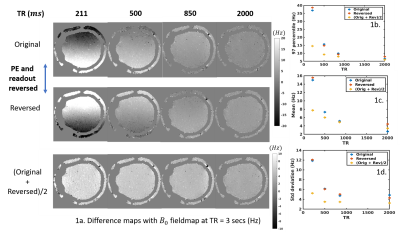 |
Characterizing the acquisition protocol dependencies of B0 field mapping and the effects of eddy currents and spoiling
Divya Varadarajan1,2, Mukund Balasubramanian2,3, Daniel J. Park1, Thomas Witzel4, Jason P. Stockmann1,2, and Jonathan R. Polimeni1,2,5
1Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 2Department of Radiology, Harvard Medical School, Boston, MA, United States, 3Boston Children’s Hospital, Boston, MA, United States, 4Qbio Inc., San Carlos, CA, United States, 5Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States
Maps of the B0 field are routinely used for MRI scanner calibration and for post-processing corrections of geometric distortions. However, several sources of bias are present in conventional field estimates, which will result in uncorrected image artifacts, yet it is unclear what the magnitude of these biases are, whether these are large enough to warrant concern, and how to reduce these errors to extract more accurate field estimates. Here we investigate the accuracy of the standard B0 field map acquisition, demonstrate that the estimated fields vary with several acquisition parameters, and investigate sources of these errors.
|
|||
3553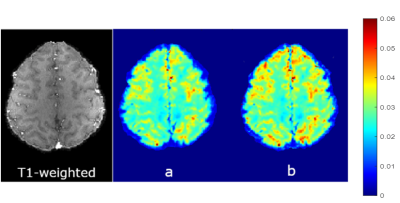 |
Application of Voxel Spread Function Method for Correction of Magnetic Field Inhomogeneity at 7T Video Permission Withheld
seyedeh nasim adnani1, Thomas Denney Jr.1, Alexander Sukstansky2, Dmitriy Yablonskiy2, and adil bashir1
1electrical and computer engineering, auburn university, auburn, AL, United States, 2radiology, Washington university school of medicine in St. Louis, St. Louis, MO, United States
Imaging in ultra-high field (7T and above) has pros and cons as compared with 3T. The pros are increases in SNR and frequency shifts that helps in separating the available biophysical compartments in the brain. Con is the increased effect of magnetic field inhomogeneity on T2* that renders the quantification unreliable. Therefore, the field correction methods are critical for ultra-high field quantitative T2* mapping. We have demonstrated the application of Voxel Spread Method, a post-processing technique, to addresses this issue at 7T. F-term correction significantly reduces magnetic field inhomogeneity effects for quantitative T2* mapping.
|
|||
3554.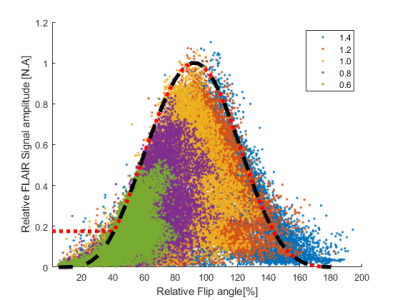 |
Correction of transmit-field induced signal inhomogeneity in 3D MP-FLAIR at 7T
Jan Ole Pedersen1, Vincent O. Boer2, Oula Puonti2, Jaco M. Zwanenburg3, and Esben Thade Petersen2,4
1Philips Healthcare, Copenhagen, Denmark, 2Danish Research Centre for Magnetic Resonance, Centre for Functional and Diagnostic Imaging and Research, Copenhagen University Hospital, Hvidovre, Denmark, 3Department of Radiology, University Medical Center Utrecht, Utrcecht, Netherlands, 4Section for Magnetic Resonance, DTU Health Tech, Technical University of Denmark, Kgs Lyngby, Denmark
In order to ease radiological assessment of 7T MP-FLAIR images, we have developed a fully automated bias-field correction that reduces apparent signal loss caused by inhomogeneities in the RF transmit field. Using simulations and measurements of the transmit field, the bias-field can be corrected for without relying on the simplifying assumptions used in a typical bias-field correction. The algorithm is expandable to other TSE-based sequences and adds limited additional scan time.
|
|||
3555.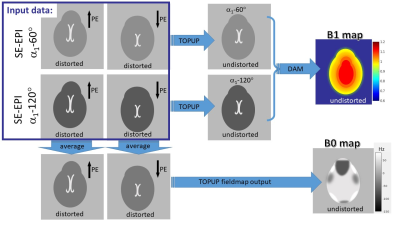 |
Integrated Spin-Echo EPI scans for Fast Simultaneous B1 and B0 mapping in the Human Brain
Sofia Chavez1,2
1Centre for Addiction and Mental Health (CAMH), Toronto, ON, Canada, 2Psychiatry, University of Toronto, Toronto, ON, Canada
Quantitative MRI requires accurate knowledge of the spatially varying B1 and B0 fields in order to accurately account for their effects on relevant parameters included in the signal models. B1 maps in the human brain are commonly produced from a double-angle method (DAM) with many variations in the implementations. B0 maps are usually estimated from the distortions in two 2D axial EPI scans acquired with opposing phase encode directions (topup). Here, we propose to integrate the SE-EPI scan requirements for B0 mapping with topup and B1 mapping with the DAM, for simultaneous B1 and B0 mapping with reduced scan time.
|
|||
3556.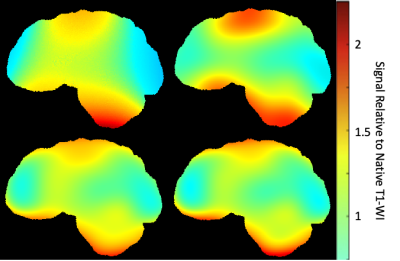 |
Optimisation of MRI Bias Field Correction Algorithms on Whole Brain and Atrophy Measurements
Kain Kyle1,2 and Chenyu Wang1,2
1Sydney Neuroimaging Analysis Centre, Sydney, Australia, 2University of Sydney, Sydney, Australia
Correction of bias fields is a common component in MRI analysis pipelines. We present a novel optimisation method to improve the white matter segmentation used for white matter weighted N4 correction, and validate using atrophy in a healthy control cohort.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.