Digital Posters
MR Fingerprinting: Sequences, Reconstruction & Applications in the Brain
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 1 | 19:00 - 20:00 |
1531.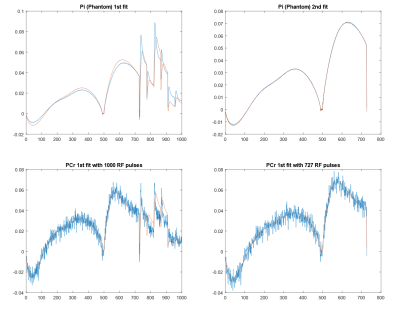 |
Rapid T1, T2 measurements and SNR evaluation by 31P MR fingerprinting in human brain at 7T
Song-I Lim1,2, Mark Stephan Widmaier1,3, Yun Jiang4, and Lijing Xin1,2
1CIBM Center for Biomedical Imaging, Lausanne, Switzerland, 2Animal Imaging and Technology, EPFL, Lausanne, Switzerland, 3Laboratory for Functional and Metabolic Imaging, EPFL,, Lausanne, Switzerland, 4Department of Radiology, University of Michigan, Ann Arbor, MI, United States
This study is to validate 31P MRS fingerprinting scheme at 7T. In this experiment, MRF scheme was validated using Pi phantom and in vivo brain data was acquired.
|
|||
1532.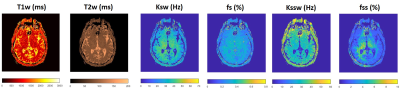 |
Development of a Clinical CEST-MR Fingerprinting (CEST-MRF) Pulse Sequence and Reconstruction Methods
Ouri Cohen1, Or Perlman2, Christian T Farrar2, and Ricardo Otazo1
1Memorial Sloan Kettering Cancer Center, New York, NY, United States, 2Radiology, Massachusetts General Hospital, Charlestown, MA, United States
Development of a CEST-MR Fingerprinting (CEST-MRF) pulse sequence combined with a physics-based deep learning approach suitable for use on a 3T clinical scanner is described and its utility demonstrated in a healthy human brain. The acquisition is short (less than 2 minutes) and simultaneously yields 6 quantitative tissue parameters that can be used for tissue characterization.
|
|||
1533.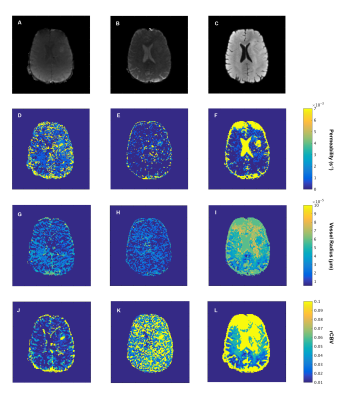 |
Vascular fingerprinting using DSC MRI for quantification of microvasculature in glioma
Krishnapriya Venugopal1, Esther A.H Warnert1, Daniëlle van Dorth2, Marion Smits1, Juan Antonio Hernandez Tamames1, Matthias J.P van Osch2, and Dirk H.J Poot1
1Radiology and Nuclear Medicine, Erasmus MC, Rotterdam, Netherlands, 2Radiology, Leiden University Medical Center, Leiden, Netherlands
This study uses a DSC based fingerprinting approach, monitoring the time evolution of a Hybrid (H) EPI sequence (HEPI) that simultaneously acquires GRE and SE. HEPI properties are incorporated from the scanner into a simulation of contrast agent extravasation and MR signal evolution. Signals simulated during bolus passage are used to construct GRE, SE and combined GRE-SE dictionaries in which vessel permeability (k), vessel radius (R), and cerebral blood volume fraction (rCBV) are varied. The dictionary is matched to in-vivo data of a brain tumor patient to retrieve information on the underlying microvasculature.
|
|||
1534.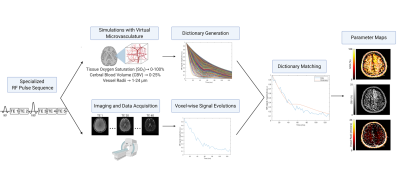 |
Noise Considerations for Accelerated MR Vascular Fingerprinting
Gregory J. Wheeler1 and Audrey P. Fan1,2
1Biomedical Engineering, University of California Davis, Davis, CA, United States, 2Neurology, University of California Davis, Davis, CA, United States
Magnetic resonance vascular fingerprinting (MRvF) enables the simultaneous measurement of quantitative oxygen saturation, cerebral blood volume, and microvascular radii maps from a single scan. Accelerating image acquisition for MRvF would enable new dynamic investigations into cerebrovascular diseases. Acquisition acceleration will result in tradeoffs between parameter map accuracy, time resolution, and noise. We performed a simulation study in which five signal-to-noise ratios and five echo train lengths were used to generate 25 simulated datasets to assess limits required for accurate matching. Vascular parameter matching accuracy increases with increased SNR and echo train length and requires an SNR above at least 20.
|
|||
 |
1535.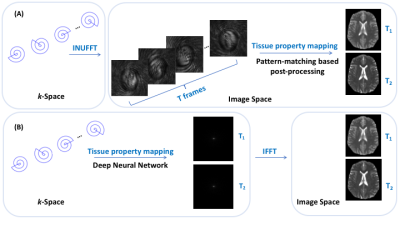 |
5-Minute MR Fingerprinting from Acquisition to Reconstruction for Whole-Brain Coverage with Isotropic Submillimeter Resolution
Yilin Liu1, Yong Chen2, and Pew-thian Yap1
1University of North Carolina at Chapel Hill, Chapel Hill, NC, United States, 2Case Western Reserve University, Cleveland, OH, United States
Existing techniques for 3D MR fingerprinting focus on accelerating either acquisition or template matching, with typically limited spatial resolution. We develop a 3D MRF sequence coupled with a novel end-to-end deep learning image reconstruction framework for rapid and simultaneous whole-brain quantification of T1 and T2 relaxation times with isotropic submillimeter spatial resolution. We demonstrate feasibility with both retrospectively and prospectively accelerated 3D MRF data.
|
||
1536.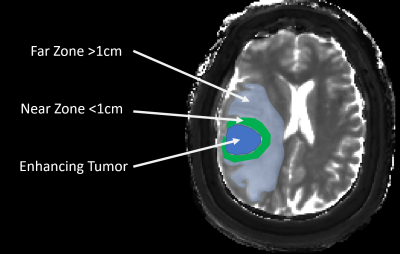 |
Differentiation of Peritumoral White Matter in Glioblastomas and Metastases using Magnetic Resonance Fingerprinting
Charit Tippareddy1, Walter Zhao2, Andrew Sloan3,4, Jeffrey Sunshine5, Jill Barnholtz-Sloan6, Mark Griswold2,5, Dan Ma2,5, and Chaitra Badve5
1Case Western Reserve University School of Medicine, Cleveland, OH, United States, 2Department of Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 3Departments of Neurosurgery and Pathology, University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 4Seidman Cancer Center and Case Comprehensive Cancer Center, Cleveland, OH, United States, 5Department of Radiology, University Hospitals Cleveland Medical Center, Cleveland, OH, United States, 6Department of Population and Quantitative Health Sciences, University Hospitals Cleveland Medical Center, Cleveland, OH, United States
The utility of MR fingerprinting (MRF) in characterization of non-enhancing tumor (NET) region in brain tumors has not been demonstrated. Quantitative characterization of NET (aka peritumoral white matter) in glioblastomas (GBM) is essential to identify imaging surrogates for tumor infiltration and predict future recurrence. Here we demonstrate the utility of pre and post contrast MRF to characterize and compare the NET region surrounding GBMs and metastases (METS). We identify NET radiomic features that are unique to each tumor type as well as features that can differentiate near (within 1 cm) versus far (beyond 1 cm) NET regions within each group.
|
|||
1537.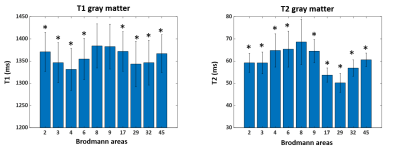 |
Exploring cyto-architecture of Brodmann areas with High-resolution 3D MR Fingerprinting
Joon Yul Yul Choi1, Siyuan Hu2, Ting-yu Su1,2, Yingying Tang1, Ken Sakaie3, Ingmar Blümcke1,4, Imad Najm1, Stephen Jones3, Mark Griswold5, Dan Ma2, and Zhong Irene Wang1
1Epilepsy Center, Neurological Institue, Cleveland Clinic, Cleveland, OH, United States, 2Biomedical Engineering, Case Western Reserve University, Cleveland, OH, United States, 3Imaging Institute, Cleveland Clinic, Cleveland, OH, United States, 4Neuropathology, University of Erlangen, Erlangen, Germany, 5Radiology, Case Western Reserve University, Cleveland, OH, United States
We demonstrate in this study the sensitivity of multi-parametric magnetic resonance fingerprinting (MRF) results at 3T to differentiate cortical regions with different cyto- or myelo-architecture. The study investigated the quantitative T1 and T2 values in various Brodmann areas to verify the sensitivity of MRF in probing tissue properties of the human cortex. Additionally, the study explores the relationship between quantitative T1 and T2 values of gray and white matter in Brodmann areas.
|
|||
1538.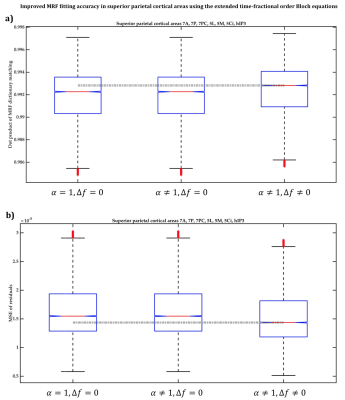 |
Human cerebral cortex parcellation using time-fractional order magnetic resonance fingerprinting (MRF)
Shahrzad Moinian1,2, David Reutens1,2, and Viktor Vegh1,2
1Centre for Advanced Imaging, The University of Queensland, Brisbane, Australia, 2ARC Training Centre for Innovation in Biomedical Imaging Technology, The University of Queensland, Brisbane, Australia We have previously shown the potential efficacy of a magnetic resonance fingerprinting (MRF) residual approach for human cerebral cortex parcellation. However, the classical Bloch equations, commonly used for MR signal simulation in the MRF dictionary, may not accurately describe the complex effect of the ensemble of microarchitectonic components of the gray matter tissue on MRF signal. This work benefitted from the more flexibility of the extended time-fractional order Bloch equations to improve MRF dictionary fitting accuracy. We demonstrated that the time-fractional order parameter α potentially associates with the effect of interareal architectonic variability, hypothetically leading to more accurate cortical parcellation. |
|||
1539.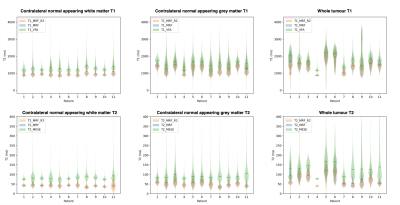 |
Validity and repeatability of MRF in glioma and normal appearing contralateral brain tissue at 3T
Simran Kukran1,2, Joely Smith3,4, Luke Dixon1,3, Ben Statton5, Sarah Cardona3, Lillie Pakzad-Shahabi6,7, Matthew Williams6,8, Dow-Mu Koh2,9, Rebecca Quest3,4, Matthew Orton2, and Matthew Grech-Sollars1,3
1Department of Surgery and Cancer, Imperial College London, London, United Kingdom, 2Department of Radiotherapy and Imaging, Institute of Cancer Research, London, United Kingdom, 3Department of Imaging, Imperial College Healthcare NHS Trust, London, United Kingdom, 4Department of Bioengineering, Imperial College London, London, United Kingdom, 5Medical Research Council, London Institute of Medical Sciences, Imperial College London, London, United Kingdom, 6Computational Oncology group, Institute for Global Health Innovation, Imperial College London, London, United Kingdom, 7John Fulcher Neuro-oncology Laboratory, Department of Brain Sciences, Imperial College London, London, United Kingdom, 8Radiotherapy Department, Charing Cross Hospital, London, United Kingdom, 9Department of Radiology, Royal Marsden Hospital, London, United Kingdom
MR Fingerprinting (MRF) was found to give highly repeatable T1 and T2 measurements in glioma and normal appearing contralateral brain tissue. Validity was investigated via comparison to standard mapping techniques: variable flip angle for T1 and multi-echo spin echo for T2. Biases were found between MRF and standard relaxometry methods, as in previous healthy volunteer studies. Statistically significant strong and moderate correlations were found between the MRF and standard mapping methods for T1 and T2 respectively, indicating MRF is comparably sensitive to changes in T1 and T2 as established mapping techniques in both cancerous and normal appearing contralateral brain tissue.
|
|||
1540.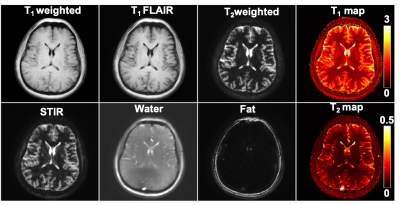 |
In vivo repeatability of Tailored MR Fingerprinting
pavan Poojar1,2, Enlin Qian1, and Sairam Geethanath 1,2
1Columbia Magnetic Resonance Research Center, Columbia University, New York, NY, United States, 2Dayananda Sagar College of Engineering, Bangalore, India
MR fingerprinting(MRF) has an advantage over quantitative MRI as it allows simultaneous acquisition of multi-parametric maps but does not generate multi-contrast images required for routine clinical studies. Tailored MRF(TMRF) allows simultaneous acquisition of two quantitative maps and six qualitative contrasts. A TMRF repeatability study was conducted for four days on one in vivo healthy human brain. Signal-to-noise ratio (SNR) and mean intensity values for grey matter(GM) and white matter(WM) were computed. Standard deviation of SNR for WM and GM were in the range of 0.1 to 0.75 and 0.2 to 1.1 respectively. This narrow range shows the repeatability of TMRF
|
|||
1541.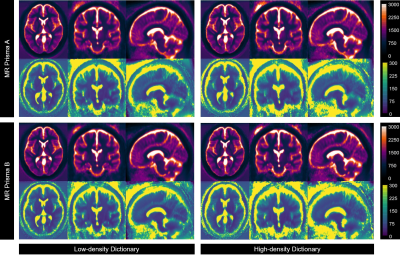 |
Reproducibility of 3D MR Fingerprinting with Different Dictionary Resolution in the Healthy Human Brain
Krishna Pandu Wicaksono1, Yasutaka Fushimi1, Satoshi Nakajima1, Akihiko Sakata1, Takuya Hinoda1, Sonoko Oshima1, Sayo Otani1, Hiroshi Tagawa1, Yang Wang1, Tomohisa Okada1, and Yuji Nakamoto1
1Kyoto University, Graduate School of Medicine, Kyoto, Japan
3D MR Fingerprinting was introduced as a rapid quantitative MRI technique, enabling higher SNR efficiency and spatial resolution than the 2D counterpart. As a crucial element of MRF reconstruction, the impact of dictionary resolution on MRF performance is essential to be investigated. Following our phantom study, which determined equivalent accuracy and repeatability of 3D MRF using two different dictionary resolutions, a reproducibility study in healthy volunteers was performed. This study demonstrated a comparable 3D MRF reproducibility from two different dictionary resolutions in most brain parenchyma. Yet, lower reproducibility was evident in CSF measurement, more obviously in a higher resolution dictionary.
|
|||
1542.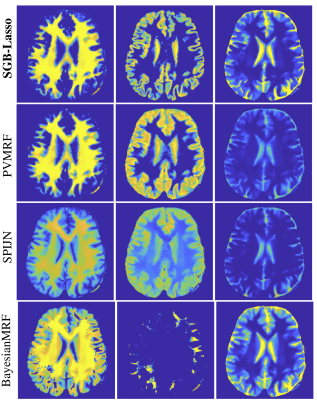 |
Multi-compartment MR Fingerprinting: an off-the-grid approach
Mohammad Golbabaee1 and Clarice Poon1
1University of Bath, Bath, United Kingdom
We introduce a novel off-the-grid sparse approximation algorithm to separate multiple tissue compartments in image voxels and to estimate quantitatively their NMR properties and mixture fractions, given the MR fingerprinting (MRF) measurements. The proposed algorithm is an accurate and importantly a scalable alternative to the multicompartment MRF baselines because it does not rely on fine-gridded multiparametric MRF dictionaries. The method is theoretically described, and its feasibility is demonstrated and compared to other baselines on in-vivo healthy brain measurements.
|
|||
1543.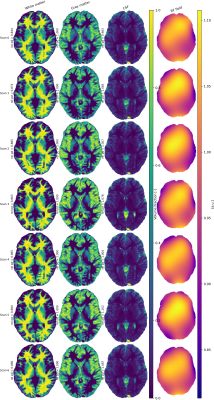 |
Estimating tissue volume fractions and proton density in multi-component MRF
Martijn A. Nagtegaal1, Laura Nunez Gonzalez2, Dirk H.J. Poot2, Matthias J.P. van Osch3, Jeroen H.J.M. de Bresser4, Juan A. Hernandez Tamames1,2, and Frans M. Vos1,2
1Department of Imaging Physics, Delft University of Technology, Delft, Netherlands, 2Department of Radiology and Nuclear Medicine, Erasmus MC, Rotterdam, Netherlands, 3C.J. Gorter Center for high field MRI, Department of Radiology, Leiden University Medical Center, Leiden, Netherlands, 4Department of Radiology, Leiden University Medical Center, Leiden, Netherlands
Accurate proton density estimations are required to obtain tissue volume fractions from multi-component MR Fingerprinting data. We propose a method for estimating relative proton densities per tissue while taking the receiver sensitivity profile into account. In 20 different numerical brain phantoms this shows to improve tissue segmentations compared to conventional methods that use $$$T_1$$$ weighted images. Estimated proton density values for single slice in vivo data (7 scans for 4 subjects) were in range with literature values in particular for white and gray matter.
|
|||
 |
1544.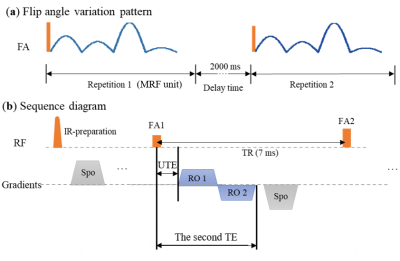 |
3D Ultrashort Echo Time MR Fingerprinting (3D UTE-MRF) for Whole Brain Myelin Imaging
Zihan Zhou1, Qing Li1,2, Congyu Liao3, Xiaozhi Cao3, Ting Gong1, Qiuping Ding1, Hongjian He1, and Jianhui Zhong1,4
1Center for Brain Imaging Science and Technology, College of Biomedical Engineering and Instrumental Science, Zhejiang University, Hangzhou, China, 2MR Collaboration, Siemens Healthcare Ltd, Shanghai, China, 3Radiological Sciences Laboratory, Stanford University, Stanford, CA, United States, 4Department of Imaging Sciences, University of Rochester, Rochester, NY, United States
We proposed a 3D ultrashort echo time MR fingerprinting (3D UTE-MRF) method for whole brain myelin imaging in vivo and ex vivo. A series of dual-echo time images were acquired, and images optimized for long T2 tissue suppression were found in the second echo image series, which were used to extract myelin signals. Non-negative least-square was used to map the ultrashort T2 myelin tissue fraction. 3D UTE-MRF sequence can achieve whole brain myelin mapping in 15 min with 0.87 mm isotropic resolution.
|
||
 |
1545.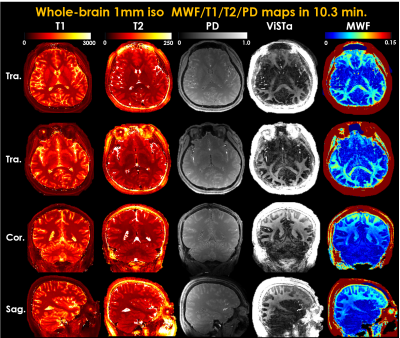 |
High-resolution myelin-water fraction (MWF) and T1/T2/proton-density mapping using 3D ViSTa-MR fingerprinting with subspace reconstruction
Congyu Liao1, Xiaozhi Cao1, Ting Gong2, Zhe Wu3, Zihan Zhou2, Hongjian He2, Jianhui Zhong2,4, and Kawin Setsompop1
1Radiological Sciences Laboratory, Stanford University, Stanford, CA, United States, 2Center for Brain Imaging Science and Technology, College of Biomedical Engineering & Instrument Science, Zhejiang University, Hangzhou, China, 3Techna Institute, University Health Network, Toronto, ON, Canada, 4Department of Imaging Sciences, University of Rochester, Rochester, NY, United States
In this work, we developed ViSTa-MRF, which combined Visualization of Short Transverse relaxation time component (ViSTa) technique with MR Fingerprinting (MRF), to achieve whole-brain myelin-water fraction (MWF) and T1/T2/PD mapping at 1mm isotropic resolution in 10 minutes on a clinical 3T scanner. To achieve this fast acquisition, the ViSTa-MRF sequence also leverages an efficient 3D-spiral-projection acquisition along with spatiotemporal subspace reconstruction. With the proposed ViSTa-MRF method, direct MWF mapping was achieved without a need for multicompartment fitting. In comparison to conventional myelin-water imaging, the ViSTa-MRF method can provide improved-SNR and faster acquisition with high image-quality.
|
||
1546.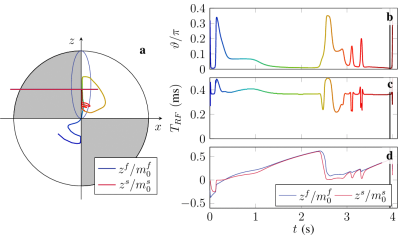 |
High Accuracy Numerical Methods for Solving Magnetic Resonance Imaging Equations and Optimizing RF Pulse Sequences
Cem Gultekin1, Jakob Assländer2, and Carlos Fernandez-Granda3
1Mathematics, Courant Institute of Mathematical Science, New York, NY, United States, 2Radiology, New York University Grossman School of Medicine, New York, NY, United States, 3Mathematics and Data Science, Courant Institute of Mathematical Science and Center for Data Science New York University, New York, NY, United States
This work presents a new robust black-box numerical solver that can reliably solve many MRI typical ordinary differential equations. Our adaptive Petrov-Galerkin (PG) method can solve challenging MRI problems with additional complexities such as B0- and B1- inhomogeneities, RF pulses, chemical exchange, and magnetization transfer (MT). We apply the proposed technique to solve an ODE-constrained optimization problem for pulse design via gradient descent. Our method reduces the time required to compute the gradients by three orders of magnitude.
|
|||
1547.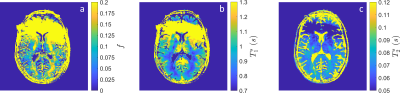 |
Myelin Water Imaging in the Hybrid State
Andrew Mao1,2,3, Sebastian Flassbeck1,2, Cem Gultekin4, Xiaoxia Zhang1,2, and Jakob Asslaender1,2
1Center for Biomedical Imaging, Department of Radiology, New York University School of Medicine, New York, NY, United States, 2Center for Advanced Imaging Innovation and Research, New York University School of Medicine, New York, NY, United States, 3Vilcek Institute of Graduate Biomedical Sciences, New York University School of Medicine, New York, NY, United States, 4Courant Institute of Mathematical Sciences, New York University, New York, NY, United States
This work extends the recently introduced hybrid-state model to myelin water imaging. We numerically optimize a hybrid-state MR fingerprinting sequence for SNR efficiency and demonstrate in vivo the ability to quantify the myelin water fraction and T2 of axonal/extra-axonal water. Our preliminary results show myelin water fraction maps in agreement with literature and demonstrate the feasibility of simultaneous myelin water fraction, T1 and T2 quantification with high spatial resolution (1.2mm isotropic), full brain coverage with a 14 minute scan.
|
|||
1548.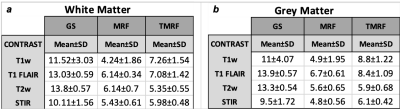 |
A faster and improved tailored Magnetic Resonance Fingerprinting
Pavan Poojar1,2, Enlin Qian1, and Sairam Geethanath 1,2
1Columbia Magnetic Resonance Research Center, Columbia University, New York, NY, United States, 2Dayananda Sagar College of Engineering, Bangalore, India
MR Fingerprinting (MRF) allows simultaneous acquisition of multi-parametric maps but the synthetic contrast images suffer from artifacts due to incomplete simulations. This work provides rapid (~4min), natural contrast (non-synthetic), quantitative (T1 and T2 maps), and qualitative images (T1-weighted, T1-FLAIR, T2-weighted, STIR, water,fat) simultaneously. Tailored MRF (TMRF) was demonstrated on four volunteers on 3T GE 750w scanner. It was compared with gold standard (GS) and MRF by computing SNR and mean intensity values of white matter (WM) and grey matter (GM) contrast. The SNR of GS>TMRF>MRF and the contrast for TMRF was greater than MRF and GS.
|
|||
1549.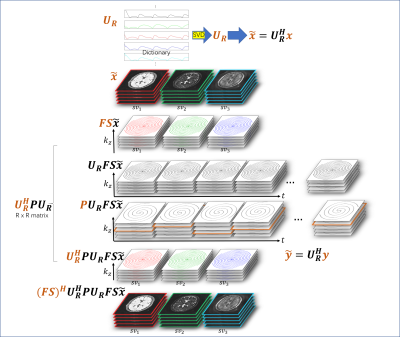 |
Iterative MR Fingerprinting Reconstruction in a Compressed k-space
Di Cui1, Edward S. Hui2, and Peng Cao1
1Diagnostic Radiology, The University of Hong Kong, Hong Kong, China, 2Rehabilitation Science, The Hong Kong Polytechnic University, Hong Kong, China
A k-space compression strategy is proposed in a 3D alternating direction method of multipliers (ADMM) framework in this study, with data and image series compressed, and intermediate computation simplified.
|
|||
1550.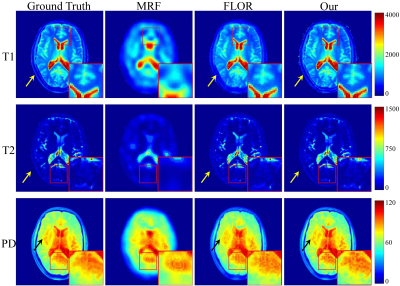 |
MR Fingerprinting Reconstruction based on Structured Low-rank Approximation and Subspace Modeling
Peng Li1 and Yue Hu1
1Harbin Institute of Technology, Harbin, China Due to the capability of fast multi-parametric quantitative imaging, magnetic resonance fingerprinting has become a promising quantitative magnetic resonance imaging (QMRI) approach. However, the highly undersampled and noise-contaminated k-space data will cause critical spatial artifacts, which subsequently lead to inaccurate estimation of the quantitative parameters. In this paper, we introduce a novel framework based on structured low-rank approximation and subspace modeling to recover temporal MRF data from its highly undersampled and noisy Fourier coefficients. |
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.