Digital Posters
Machine Learning for Data Acquisition & Image Reconstruction
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 1 | 13:00 - 14:00 |
1749. |
Adapting the U-net for Multi-coil MRI Reconstruction
Makarand Parigi1, Abhinav Saksena1, Nicole Seiberlich2, and Yun Jiang2
1Computer Science and Engineering, University of Michigan, Ann Arbor, MI, United States, 2Department of Radiology, University of Michigan, Ann Arbor, MI, United States
We propose a U-net based architecture for multi-coil MRI reconstruction. The model is able to utilize the multi-coil nature of the data by processing images before they are combined, unlike U-nets which require coil combination before inputting the image. We achieved SSIM scores higher than the U-net with much fewer parameters, enabling lower memory usage.
|
|||
1750.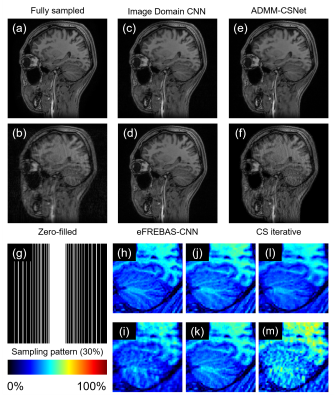 |
Multi-channel and multi-group-based CNN Image Reconstruction using Equal-sized Multi-resolution Image Decomposition
Shohei Ouchi1 and Satoshi ITO1
1Utsunomiya University, Utsunomiya, Japan
CNN based Compressed Sensing reconstruction has attracted much attention. It is difficult to restore higher spatial frequency components even with CNN-CS. We proposed a new transformed image domain CNN-CS method based on equal-sized multi-resolution image decomposition (eFREBAS transform). The eFREBAS transform is multi resolution analysis method that divide the image into equal-sized sub-images. This CNN consisting of three U-Net CNNs, each with multi-channel input and output reconstructs MR phase varied images in frequency band-to-band through estimating artifact-free sub-images from under-sampled sub-images. Reconstruction experiments showed that eFREBAS-CNN could reconstruct sharp images that have strong phase variation.
|
|||
1751.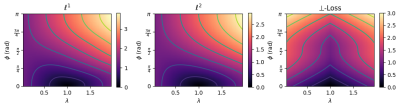 |
Rethinking complex image reconstruction: ⟂-loss for improved complex image reconstruction with deep learning
Maarten Terpstra1,2, Matteo Maspero1,2, Jan Lagendijk1, and Cornelis A.T. van den Berg1,2
1Department of Radiotherapy, University Medical Center Utrecht, Utrecht, Netherlands, 2Computational Imaging Group for MR diagnostics & therapy, University Medical Center Utrecht, Utrecht, Netherlands
The \(\ell^2\) norm is the default loss function for complex image reconstruction. In this work, we investigate the behavior of the \(\ell^1\) and \(\ell^2\) loss functions for complex image reconstruction with non-complex-valued models. Simulations show that these norms assign a lower loss to reconstructions with lower magnitude, introducing an asymmetry in the loss function. To address this, we propose a new, symmetric loss function, and train deep learning models to show that the proposed loss function achieves better performance and faster convergence on complex image reconstruction tasks.
|
|||
1752.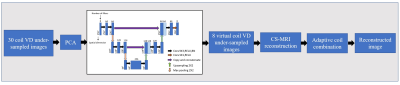 |
PCA and U-Net based Channel Compression for Fast MR Image Reconstruction
Madiha Arshad1, Mahmood Qureshi1, Omair Inam1, and Hammad Omer1
1Medical Image Processing Research Group (MIPRG), Department of Electrical and Computer Engineering, COMSATS University, Islamabad, Pakistan
In parallel imaging, the array coil with a large number of channels accelerates the data acquisition speed and provides high quality reconstructed images at the cost of an increase in computational complexity, image reconstruction time and memory requirement. Recently, Principal Component Analysis (PCA) has been used to compress multiple physical channels to a few virtual channels. However, the results showed a degradation in image quality and loss of sensitivity information. We introduce a novel channel compression technique combining PCA and U-Net before Compressed Sensing MRI reconstruction. The experimental results show less channel compression losses and retention of coil sensitivity information.
|
|||
1753.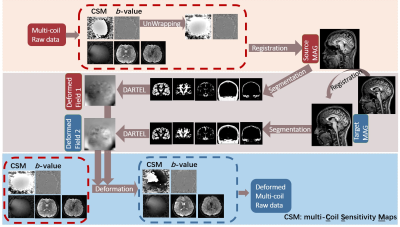 |
MAGnitude Image to Complex (MAGIC)-Net: reconstructing multi-coil complex images with pre-trained network using synthesized raw data
Fanwen Wang1, Hui Zhang1, Jiawei Han1, Fei Dai1, Yuxiang Dai1, Weibo Chen2, Chengyan Wang3, and He Wang1,3
1Institute of Science and Technology for Brain-Inspired Intelligence, Fudan University, Shanghai, China, 2Philips Healthcare, Shanghai, China, 3Human Phenome Institute, Fudan University, Shanghai, China
This study proposed a novel MAGnitude Image to Complex (MAGIC) Network to reconstruct images using deep learning with limited number of training data. Collecting complex multi-coil data is inconvenient since it is beyond the routine examination. However, there are many magnitude images available in hospitals. By applying deformation between the magnitude image and complex image, MAGIC Net succeeded in synthesizing deformed data for training and enabled deep learning methods. Results show that with the same original data, MAGIC-Net outperforms the conventional CG-SENSE in PSNR for all undersampling trajectories with high resolution b = 0 and b = 1000 s/mm2.
|
|||
1754.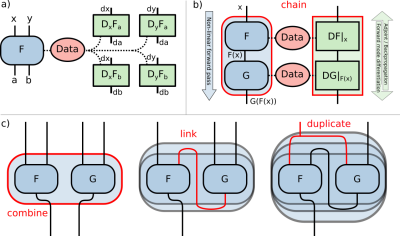 |
Deep, Deep Learning with BART
Moritz Blumenthal1 and Martin Uecker1,2,3,4
1Institute for Diagnostic and Interventional Radiology, University Medical Center Göttingen, Göttingen, Germany, 2DZHK (German Centre for Cardiovascular Research), Göttingen, Germany, 3Campus Institute Data Science (CIDAS), University of Göttingen, Göttingen, Germany, 4Cluster of Excellence “Multiscale Bioimaging: from Molecular Machines to Networks of Excitable Cells” (MBExC), University of Göttingen, Göttingen, Germany
Deep learning offers powerful tools for enhancing image quality and acquisition speed of MR images. Standard frameworks such as TensorFlow and PyTorch provide simple access to deep learning methods. However, they lack MRI specific operations and make reproducible research and code reuse more difficult due to fast changing APIs and complicated dependencies. By integrating deep learning into the MRI reconstruction toolbox BART, we have created a reliable framework combining state-of-the-art MRI reconstruction methods with neural networks. For demonstration, we reimplemented the Variational Network and MoDL. Both implementations achieve similar performance as implementations using TensorFlow.
|
|||
1755.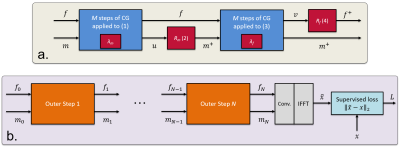 |
Deep J-Sense: An unrolled network for jointly estimating the image and sensitivity maps
Marius Arvinte1, Sriram Vishwanath1, Ahmed H Tewfik1, and Jonathan I Tamir1,2,3
1Electrical and Computer Engineering, The University of Texas at Austin, Austin, TX, United States, 2Diagnostic Medicine, Dell Medical School, The University of Texas at Austin, Austin, TX, United States, 3Oden Institute for Computational Engineering and Sciences, The University of Texas at Austin, Austin, TX, United States
Accurate reconstruction using parallel imaging relies on estimating a set of sensitivity maps from a fully-sampled calibration region, which can lead to reconstruction artifacts in poor signal-to-noise ratio conditions. We introduce Deep J-Sense as a deep learning approach for jointly estimating the image and the sensitivity maps in the frequency-domain. We formulate an alternating minimization problem that uses convolutional neural networks for regularization and train the unrolled model end-to-end. We compare reconstruction performance with model-based deep learning methods that only optimize the image and show that our approach is superior.
|
|||
1756. |
Using data-driven image priors for image reconstruction with BART
Guanxiong Luo1, Moritz Blumenthal1, and Martin Uecker1,2
1Institute for Diagnostic and Interventional Radiology, University Medical Center Göttingen, Germany, Göttingen, Germany, 2Campus Institute Data Science (CIDAS), University of Göttingen, Germany, Göttingen, Germany
The application of deep learning has is a new paradigm for MR image reconstruction. Here, we demonstrate how to incorporate trained neural networks into pipelines using reconstruction operators already provided by the BART toolbox. As a proof of concept, we demonstrate how to incorporate a deep image prior trained via TensorFlow into reconstruction within BART's framework.
|
|||
1757.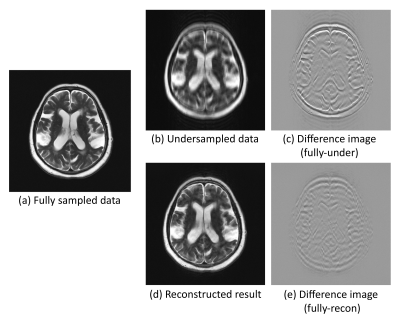 |
Going beyond the image space: undersampled MRI reconstruction directly in the k-space using a complex valued residual neural network
Soumick Chatterjee1,2,3, Chompunuch Sarasaen1,4, Alessandro Sciarra1,5, Mario Breitkopf1, Steffen Oeltze-Jafra5,6,7, Andreas Nürnberger2,3,7, and Oliver Speck1,6,7,8
1Department of Biomedical Magnetic Resonance, Otto von Guericke University, Magdeburg, Germany, 2Data and Knowledge Engineering Group, Otto von Guericke University, Magdeburg, Germany, 3Faculty of Computer Science, Otto von Guericke University, Magdeburg, Germany, 4Institute of Medical Engineering, Otto von Guericke University, Magdeburg, Germany, 5MedDigit, Department of Neurology, Medical Faculty, University Hopspital, Magdeburg, Germany, 6German Centre for Neurodegenerative Diseases, Magdeburg, Germany, 7Center for Behavioral Brain Sciences, Magdeburg, Germany, 8Leibniz Institute for Neurobiology, Magdeburg, Germany
One of the common problems in MRI is the slow acquisition speed, which can be solved using undersampling. But this might result in image artefacts. Several deep learning based techniques have been proposed to mitigate this problem. Most of these methods work only in the image space. Fine anatomical structures obscured by artefacts in the image can be challenging to reconstruct for a model working in the image space, but not in k-space. In this research, a novel complex-valued ResNet has been proposed to work directly in the k-space to reconstruct undersampled MRI. The preliminary experiments have shown promising results.
|
|||
1758.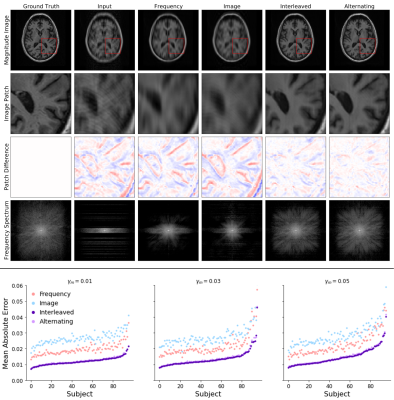 |
A Deep-Learning Framework for Image Reconstruction of Undersampled and Motion-Corrupted k-space Data
Nalini M Singh1,2, Juan Eugenio Iglesias1,3,4, Elfar Adalsteinsson2,5,6, Adrian V Dalca1,4, and Polina Golland1,5,6
1Computer Science and Artificial Intelligence Laboratory, Massachusetts Institute of Technology, Cambridge, MA, United States, 2Harvard-MIT Division of Health Sciences and Technology, Massachusetts Institute of Technology, Cambridge, MA, United States, 3Centre for Medical Image Computing, University College London, London, United Kingdom, 4A. A. Martinos Center for Biomedical Imaging, Department of Radiology, Massachusetts General Hospital, Charlestown, MA, United States, 5Department of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 6Institute for Medical Engineering and Science, Massachusetts Institute of Technology, Cambridge, MA, United States
We propose a deep learning approach for reconstructing undersampled k-space data corrupted by motion. Our algorithm achieves high-quality reconstructions by employing a novel neural network architecture that captures the correlation structure jointly present in the frequency and image spaces. This method provides higher quality reconstructions than techniques employing solely frequency space or solely image space operations. We further characterize the motion severities for which the proposed method is successful. This analysis represents the first step toward fast image reconstruction in the presence of substantial motion, such as in pediatric or fetal imaging.
|
|||
1759.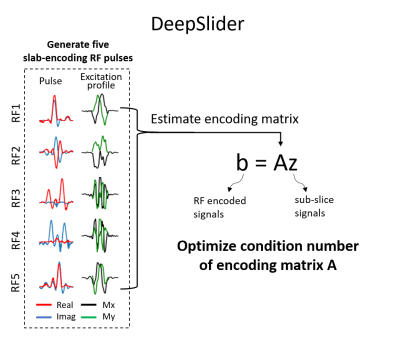 |
DeepSlider: Deep learning-powered gSlider for improved robustness and performance
Juhyung Park1, Dongmyung Shin1, Hyeong-Geol Shin1, Jiye Kim1, and Jongho Lee1
1Seoul National University, Seoul, Korea, Republic of
We designed a slab-encoding RF pulse set, referred to as DeepSlider, using deep reinforcement learning to improve the robustness and performance. The condition number of the RF encoding matrix, which determines the sensitivity of the design to noise, was optimized via deep reinforcement learning and gradient descent. Additionally, the design was extended from reconstructing the real signal to the complex signal, allowing us to utilize the complex signal instead of the real part, expanding the application of the pulse to phase-based imaging.
|
|||
1760.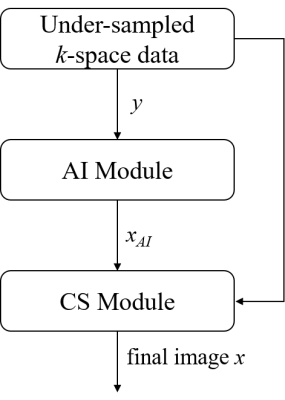 |
Intelligent Incorporation of AI with Model Constraints for MRI Acceleration
Renkuan Zhai1, Xiaoqian Huang2, Yawei Zhao1, Meiling Ji1, Xuyang Lv2, Mengyao Qian1, Shu Liao2, and Guobin Li1
1United Imaging Healthcare, Shanghai, China, 2United Imaging Intelligence, Shanghai, China
The advantages of Convolutional Neural Networks (CNN) for MRI acceleration have been widely reported, but one remaining problem is that the significantly complex network makes itself less explainable than conventional model-based methods. In this work, a novel deep learning assisted MRI acceleration method is introduced to address the uncertainty of CNN by integrating its output as another constraint into the framework of Compressed Sensing (CS).
|
|||
1761.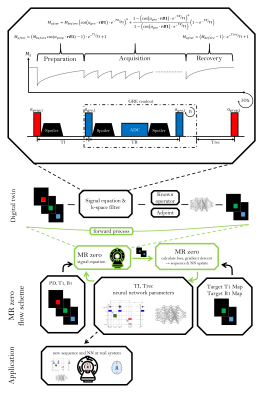 |
MRzero sequence generation using analytic signal equations as forward model and neural network reconstruction for efficient auto-encoding
Simon Weinmüller1, Hoai Nam Dang1, Alexander Loktyushin2,3, Felix Glang2, Arnd Doerfler1, Andreas Maier4, Bernhard Schölkopf3, Klaus Scheffler2,5, and Moritz Zaiss1,2
1Neuroradiology, University Clinic Erlangen, Friedrich-Alexander Universität Erlangen-Nürnberg (FAU), Erlangen, Germany, 2Max-Planck Institute for Biological Cybernetics, Magnetic Resonance Center, Tübingen, Germany, 3Max-Planck Institute for Intelligent Systems, Empirical Inference, Tübingen, Germany, 4Pattern Recognition Lab Friedrich-Alexander-University Erlangen-Nürnberg, Erlangen, Germany, 5Department of Biomedical Magnetic Resonance, Eberhard Karls University Tübingen, Tübingen, Germany
MRzero is a fully differentiable Bloch-equation-based MRI sequence invention framework. Instead of using time-consuming average-isochromat-based Bloch simulations, analytic signal equations are used as alternative forward differentiable MR scan simulation method. Neural network reconstruction is used for efficient auto-encoding. The joint optimization of sequence and NN parameters for B1 and T1 mapping can be performed 2 to 3 orders of magnitude faster then in previous MRzero approaches. The optimized sequence is tested by measurements in vivo at 3T and compared to a standard inversion recovery. High quality B1 and T1 maps are provided with less total acquisition time and energy deposition.
|
|||
1762.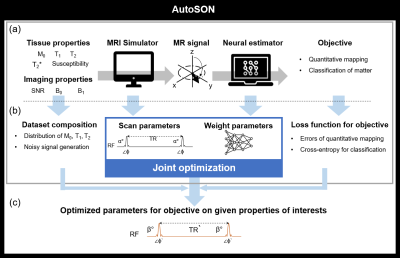 |
AutoSON: Automated Sequence Optimization by joint training with a Neural network
Hongjun An1, Dongmyung Shin1, Hyeong-Geol Shin1, Woojin Jung1, and Jongho Lee1
1Department of Electrical and computer Engineering, Seoul National University, Seoul, Korea, Republic of
In MRI, scan parameters are carefully selected for desired results. We propose a new optimization method, AutoSON, that determines optimal scan parameters for flexibly-selected objectives on given tissue properties such as distribution and noises. AutoSON optimizes not only scan parameters but also a neural estimator, which extracts the desired information from MR signals (e.g., quantification mapping). The method successfully optimized the flip angles in DESPOT1 for T1 mapping and classification of white matter and gray matter. The last example does not have a simple analytic equation, and therefore, demonstrates a potential utility of the method.
|
|||
1763.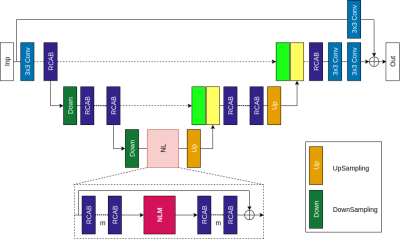 |
Deep Learning Based Joint MR Image Reconstruction and Under-sampling Pattern Optimization
Vihang Agarwal1, Yue Cao1, and James Balter1
1Radiation Oncology, University of Michigan, Ann Arbor, MI, United States
Accelerating MRI acquisition by under-sampling measurements in k-space and learning an image reconstruction model with high image quality is necessary to expand its clinical utilization. In this work, we explore joint optimization of under-sampling patterns and image reconstruction neural networks for aggressive sub-sampling of images that require very long acquisition times (e.g., FLAIR T2 weighted images). We propose Attention Residual Non-Local Networks (ARNL-Net) trained with an uncertainty based L1 loss function for producing high quality images. Initial experiments demonstrate the practicability of this method, with reconstructions demonstrating superior fidelity to fully sampled images as compared to random under-sampling schemes.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.