Oral
Quantitative Cardiovascular Tissue Characterization
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 3 | 12:00 - 14:00 | Moderators: Mehmet Akcakaya & Bettina Baeßler |
0685.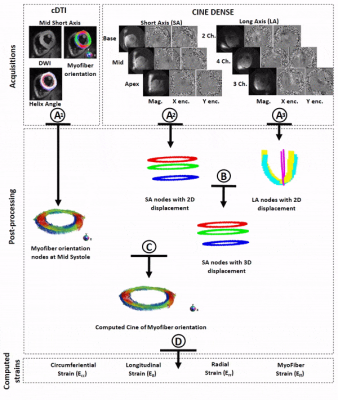 |
Myofiber strain in healthy humans using cDTI and Cine DENSE MRI
Kevin Moulin1,2,3, Pierre Croisille4,5, Magalie Viallon4,5, Ilya A Verzhbinsky6, Luigi E Perotti7, and Daniel B Ennis1,2,3
1Department of Radiology, Stanford University, Stanford, CA, United States, 2Department of Radiology, Veterans Administration Health Care System, Palo Alto, CA, United States, 3Cardiovascular Institute, Stanford University, Stanford, CA, United States, 4University of Lyon, UJM-Saint-Etienne, INSA, CNRS UMR 5520, INSERM U1206, CREATIS, F-42023, Saint-Etienne, France, 5Department of Radiology, University Hospital Saint-Etienne, Saint-Etienne, France, 6Medical Scientist Training Program, University of California - San Diego, La Jolla, CA, United States, 7Department of Mechanical and Aerospace Engineering, University of Central Florida, Orlando, FL, United States
Despite the importance of myofiber strain (Eff) to overall heart function, it has remained very difficult to measure Eff in vivo owing to the challenges of measuring both microstructural and functional cardiac data. We propose a new method that integrates cDTI and a volume of short- and long-axis DENSE slices with 2D displacement encoding to enable the measurement of in vivo Eff in humans. The accuracy of the approach for measuring Eff was evaluated in silico. Finally, in vivo Eff values were measured and reported for thirty (N=30) healthy volunteers for which an average Eff=-0.14 was found.
|
||
0686.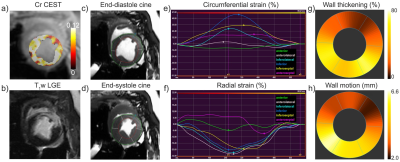 |
Feasibility of creatine chemical exchange saturation transfer (CEST) imaging in evaluating cardiac dysfunction in acute infarct heart
Yin Wu1, Jie Liu1, Qi Liu2, Hui Liu2, Jian Xu2, Yuanwei Xu3, Yucheng Chen3, Xin Liu1, and Hairong Zheng1
1Paul C. Lauterbur Research Center for Biomedical Imaging, Shenzhen Institutes of Advanced Technology, Chinese Academy of Sciences, Shenzhen, China, 2United Imaging Healthcare America, Houston, TX, United States, 3Cardiology Division, West China Hospital, Sichuan University, Chengdu, China
This study aims to investigate the feasibility of creatine (Cr) CEST imaging in assessing cardiac contractile function impairment. Eleven MI pigs underwent cine, Cr CEST and LGE imaging at 3T. Significant reduction of Cr CEST and function indices (i.e., CS, RS, WT and WM) was shown in infarct myocardium compared to that in the remote region. Cardiac function indices were shown to decrease with Cr CEST signal with moderate correlations (P<0.001). The study demonstrated the intrinsic linkage between creatine metabolic and functional changes in MI heart, suggesting the feasibility of Cr CEST in evaluating cardiac dysfunction at the molecular level.
|
||
 |
0687.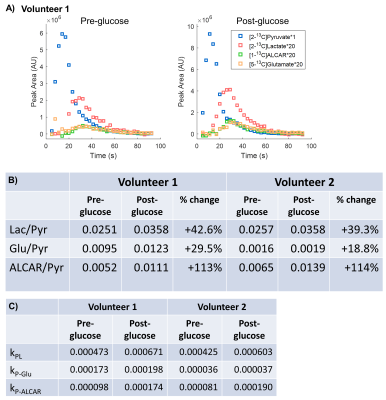 |
Probing Human Myocardial Krebs Cycle Metabolism and Response to Glucose Challenge using Hyperpolarized [2-13C]Pyruvate MR Spectroscopy
Hsin-Yu Chen1, Jeremy W. Gordon1, Nicholas Dwork1, Brian T. Chung1, Andrew Riselli2, Robert A. Bok1, James B. Slater1, M. Roselle Abraham3, Daniel B. Vigneron1, and Peder E.Z. Larson1
1Radiology and Biomedical Imaging, University of California, San Francisco, San Francisco, CA, United States, 2School of Pharmacy, University of California, San Francisco, San Francisco, CA, United States, 3Department of Medicine-Cardiology, University of California, San Francisco, San Francisco, CA, United States
This study explored the safety and feasibility to visualize real-time myocardial Krebs cycle energetics in healthy volunteers using hyperpolarized [2-13C]pyruvate MR spectroscopy, and to investigate the response to oral glucose challenge. Identified metabolic products included [2-13C]lactate, Krebs cycle-related intermediate [5-13C]glutamate, and [1-13C]acetylcarnitine, a key player in the “carnitine shuttle” of mitochondrial fatty acid oxidation. Upon oral glucose challenge, the levels of all three products increased, illustrating the metabolic flexibility of human heart to switch between fatty acid and carbohydrates.
|
|
 |
0688.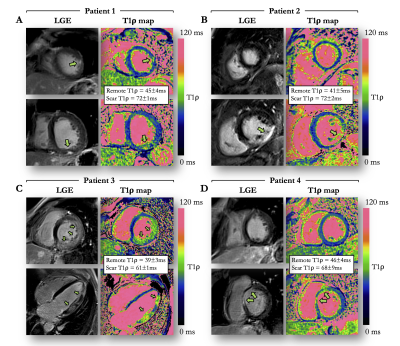 |
Single-shot model-based non-rigid motion-corrected T1 rho mapping for endogenous assessment of myocardial injury
Aurelien Bustin1,2,3, Soumaya Sridi2, Solenn Toupin4, Jerome Yerly3,5, Davide Piccini3,6, Ruud B van Heeswijk3, Pierre Jaïs1,7, Hubert Cochet1,2, and Matthias Stuber1,3,5
1IHU LIRYC, Electrophysiology and Heart Modeling Institute, Université de Bordeaux, INSERM, Centre de recherche Cardio-Thoracique de Bordeaux, U1045, Bordeaux, France, 2Department of Cardiovascular Imaging, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France, 3Department of Diagnostic and Interventional Radiology, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland, 4Siemens Healthcare France, Saint-Denis, France, 5Center for Biomedical Imaging (CIBM), Lausanne, Switzerland, 6Advanced Clinical Imaging Technology, Siemens Healthcare, Lausanne, Switzerland, 7Department of Cardiac Electrophysiology, Hôpital Cardiologique du Haut-Lévêque, CHU de Bordeaux, Bordeaux, France
Magnetic resonance T1 rho mapping may detect myocardial injuries without the need for exogenous contrast agents. However, multiple and differently T1 rho weighted co-registered acquisitions are required, and the lack of robust motion correction limits its clinical translation. This study introduces a novel automated model-based non-rigid motion correction technique for myocardial T1 rho mapping that makes use of the known signal model to drive the motion correction process. The performance, efficiency and clinical feasibility of the developed framework was investigated prospectively in a cohort of 30 patients with a broad range of ischemic and non-ischemic cardiomyopathies.
|
|
 |
0689.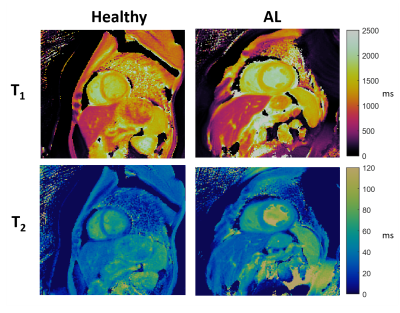 |
Characterization of Cardiac Amyloidosis using Cardiac Magnetic Resonance Fingerprinting: Preliminary Results
Brendan L Eck1, Nicole Seiberlich2, Scott D Flamm1,3, Jesse I Hamilton2, Mazen Hanna3, Yash Kumar4, Abhilash Suresh3, Angel Lawrence1,3, W. H. Wilson Tang3, and Deborah Kwon3
1Imaging Institute, Cleveland Clinic, Cleveland, OH, United States, 2Radiology, University of Michigan, Ann Arbor, MI, United States, 3Heart and Vascular Institute, Cleveland Clinic, Cleveland, OH, United States, 4Case Western Reserve University, Cleveland, OH, United States
Cardiac amyloidosis is an infiltrative cardiomyopathy characterized by the accumulation of misfolded proteins in the myocardium. Elevated myocardial T1 and T2 have been reported as a potential biomarker of disease. Cardiac Magnetic Resonance Fingerprinting (cMRF) has the potential to provide improved tissue characterization for cardiac amyloidosis through simultaneous T1 and T2 mapping. Furthermore, signal evolutions obtained by cMRF may enable improved tissue characterization. In this preliminary study of cardiac amyloidosis patients, relaxometric quantities and signal evolution data are analyzed. Myocardial T1 and T2 were elevated in patients, and linear discriminant analysis of signal evolution data suggests improved discrimination of disease.
|
|
 |
0690.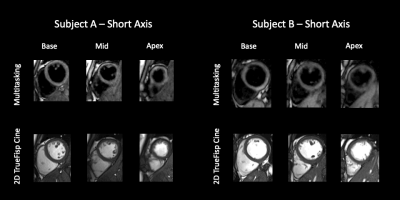 |
3D Whole-ventricle, Free-Breathing, Non-ECG, T1-T2-B1+ Mapping and Cine Imaging with Cardiac MR Multitasking
Xianglun Mao1, Fardad M Serry1, Sen Ma1, Zhehao Hu1,2, Alan C Kwan1,3, Fei Han4, Yibin Xie1, Debiao Li1,2, and Anthony G Christodoulou1,2
1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 2Department of Bioengineering, University of California in Los Angeles, Los Angeles, CA, United States, 3Smidt Heart Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States, 4Siemens Medical Solutions Inc., Los Angeles, CA, United States
Conventional cardiovascular MR (CMR) exams are relatively inefficient and demanding for patients because 1) they rely on methods that necessitate breath-holding or intermittent pauses (via gating) to compensate for motion, 2) images are acquired as a series of 2D slices often with large gaps. We propose a single 3D free-breathing acquisition without ECG requirements, providing motion resolved, quantitative T1 and T2 cine mapping with whole-ventricle coverage with high resolution and no slice gaps. The 3D Multitasking framework additionally incorporates a B1+ component, critical for accurate T1 measurement at 3T. This technique is preliminarily validated both in phantoms and healthy volunteers.
|
|
 |
0691.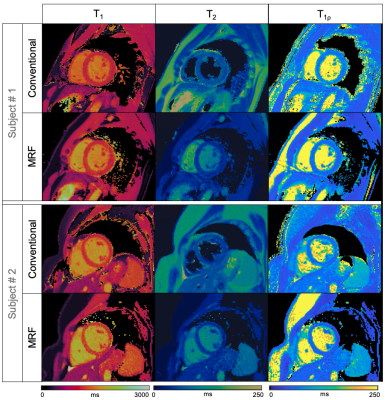 |
Simultaneous T1, T2 and T1ρ cardiac Magnetic Resonance Fingerprinting for Contrast-free Myocardial Tissue Characterization
Carlos Velasco1, Gastao Cruz1, René M. Botnar1, and Claudia Prieto1
1School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Cardiac Magnetic Resonance Fingerprinting (MRF) has shown promising results for myocardial fibrosis and inflammation characterization. In addition, T1ρ mapping has shown promising results for detection of focal and diffuse myocardial fibrosis without the need of exogenous contrast agents. However, multiparametric T1, T2 and T1ρ mapping requires sequential acquisitions under several breath-holds, that can lead to non-registered maps and bias due to inter-parameter dependencies. In this work we propose a cardiac MRF acquisition scheme for simultaneous quantification of myocardial T1, T2 and T1ρ in a contrast-free single breath-hold MR scan. The proposed approach has been investigated in phantoms and healthy subjects.
|
|
0692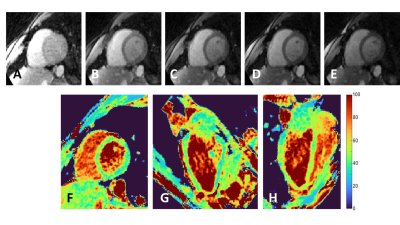 |
Fast high-resolution isotropic whole-heart T2 mapping using focused navigation Video Permission Withheld
Simone Rumac1, Christopher W. Roy2, Jérôme Yerly2,3, John Heerfordt2,4, Davide Piccini2,4, Matthias Stuber3,5, and Ruud B. van Heeswijk2
1Department of Radiology, Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Laus, Lausanne, Switzerland, 2Department of Radiology, Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne (UNIL), Lausanne, Switzerland, Lausanne, Switzerland, 3CIBM Center for BioMedical Imaging, Lausanne, Switzerland, Lausanne, Switzerland, 4Advanced Clinical Imaging Technology, Siemens Healthcare AG, Lausanne, Switzerland, Lausanne, Switzerland, 5Department of Radiology, Department of Radiology, Lausanne University Hospital (CHUV) and University of Lausanne, Lausanne, Switzerland
Cardiac parametric mapping techniques are gaining traction for the clinical routine assessment of various pathologies. Despite the complex 3D patterns of many myocardial conditions, most current techniques are breath-held single-slice 2D acquisitions. We propose a free-breathing high-resolution isotropic 3D T2 mapping technique for the heart where breathing motion is corrected in k-space before image reconstruction. In 4 healthy volunteers and one patient with myocardial infarction, we found that our technique produced sharp and accurate T2 maps but had slightly lower precision than routine techniques.
|
||
 |
0693.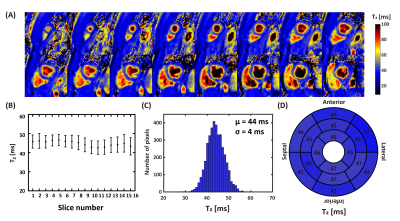 |
Single breath-holding three-dimensional cardiac T2 mapping with low-rank plus sparsity reconstruction
Dongyue Si1, Shuo Chen1, Daniel A. Herzka2, and Haiyan Ding1
1Center for Biomedical Imaging Research, Department of Biomedical Engineering, Tsinghua University, Beijing, China, 2National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States
Three-dimensional (3D) T2 mapping techniques enables quantitative detection of edematous tissue with whole heart coverage. However, the intrinsically long scan time limits its clinical application. In this study an accelerated 3D T2 mapping sequence was developed based on low-rank plus sparsity reconstruction. Both retrospective and prospective experiments were performed to evaluate the accuracy and precision of the proposed method. Achieved image quality was comparable with 4 times acceleration. Homogeneous whole left ventricular T2 map can be acquired in single breath-hold with resolution of 2×2×5 mm3.
|
|
 |
0694.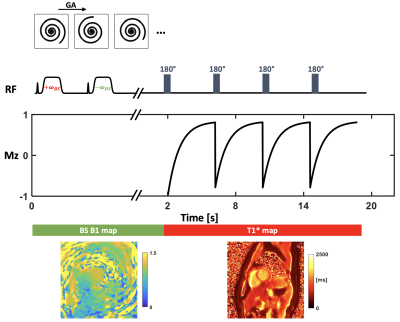 |
Comparison of free-breathing self-gated continuous IR spiral T1 mapping: dual flip angle versus Bloch-Siegert B1-corrected techniques
Ruixi Zhou1, Daniel S. Weller2, Yang Yang3, Junyu Wang1, John P. Mugler4, and Michael Salerno5
1Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 2Electrical and Computer Engineering, University of Virginia, Charlottesville, VA, United States, 3Biomedical Engineering and Imaging Institute and Department of Radiology, Icahn School of Medicine at Mount Sinai, New York, NY, United States, 4Radiology, Biomedical Engineering, University of Virginia, Charlottesville, VA, United States, 5Cardiology, Radiology, Biomedical Engineering, University of Virginia, Charlottesville, VA, United States
We propose a technique to acquire accurate B1 and T1 maps in a free-breathing cardiac self-gated continuous Look-Locker, inversion-recovery acquisition. Data are acquired using a single spiral interleaf, rotated by the golden-angle in time. During the first 2 seconds, off-resonance Fermi pulses are applied to generate a Bloch-Siegert shift B1 map, and the later data are acquired with an inversion RF pulse applied every four seconds to create T1* map. The final T1 map is generated with the B1 map and T1* map by using a look-up table to account for slice profile effects yielding more accurate T1 values.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.