Oral
Quantitative Relaxation Parameter Mapping in the Brain
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 1 | 16:00 - 18:00 | Moderators: Karin Shmueli & Vanessa Wiggermann |
0549.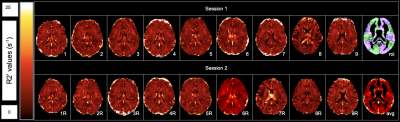 |
Multi-parametric R2’ Measurement of Brain Oxygen Extraction Fraction: Reproducibility and Application in Moyamoya Disease
Matthew Kim1, Denise Zhong1, Moss Y Zhao2, David Y.T Chen3, David D Shin4, Greg Zaharchuk2, and Audrey P. Fan1,5
1Department of Biomedical Engineering, University of California, Davis, Davis, CA, United States, 2Department of Radiology, Stanford University, Stanford, CA, United States, 3Department of Medical Imaging, Taipei Medical University-Shuan-Ho Hospital, New Taipei City, Taiwan, 4General Electric Healthcare, San Ramon, CA, United States, 5Department of Neurology, University of California Davis, Davis, CA, United States
We quantified R2’ as a biomarker of brain oxygen extraction fraction (OEF) using a multi-parametric approach, based on multi-echo gradient echo (R2*) and fast spin echo scans (R2). Healthy volunteers received repeat scans after 1-2 weeks and the coefficient of variation of R2’ was low (4.4-8.3% in vascular territories), indicating good R2’ scan-rescan reproducibility. R2’ was also inversely correlated with PET scans of cerebral blood flow in healthy controls, as expected. In 14 Moyamoya patients, elevated R2’ (indicating abnormal, high OEF) was observed both in brain regions with and without artery stenosis, and provided complementary information to cerebral perfusion.
|
||
 |
0550.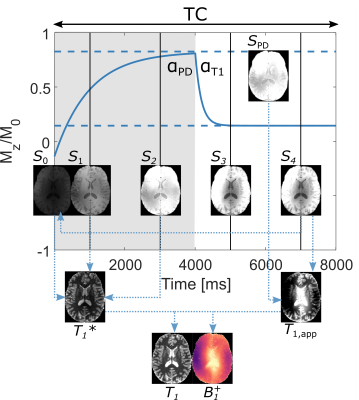 |
Simultaneous 3D T1 and B1+ mapping at 7T using MPRAGE with multiple volumes and driven equilibrium (DE)
Hampus Olsson1, Mads Andersen2, Mustafa Kadhim1, and Gunther Helms1
1Department of Medical Radiation Physics, Clinical Sciences Lund, Lund University, Lund, Sweden, 2Philips Healthcare, Copenhagen, Denmark
MP2RAGE has become popular for T1 mapping at 7T. Accuracy is yet improved by using a separately acquired flip angle map when creating the protocol-specific lookup table. Here, two additional volumes acquired at differing flip angles are added to an MP2RAGE sequence to obtain two separate states of driven equilibriums, effectively forming a dual flip angle protocol within the cycle. By estimating the accelerated effective relaxation, T1*, from the signals, both T1 and B1+ can be solved for analytically. Thus, a multi-volume MPRAGE sequence is turned into a dedicated high-resolution T1 and flip angle-mapping protocol.
|
|
0551.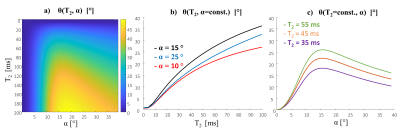 |
STARE (Steady-state T2 And Rf Estimation) - A fast 3D-GRE acquisition for phase-based mapping of T2 and B1
Rita Schmidt1,2, Amir Seginer3, and Yael Kierson1,2
1Neurobiology, Weizmann Institute of Science, Rehovot, Israel, 2The Azrieli National Institute for Human Brain Imaging and Research, Weizmann Institute of Science, Rehovot, Israel, 3Siemens Healthcare Ltd, Rosh Ha’ayin, Israel
There is a bulk of studies exploring spin-echo and multi-echo methods generating T2 weighted imaging and T2 mapping. However, the challenges at 7T MRI include high SAR, long scans and RF inhomogeneity. Steady-state GRE also provide T2 maps based on the magnitude images. A recent study at 3T demonstrated phase-based 3D-GRE with specific RF phase increments producing T2 maps. We propose acquiring four scans from which we can estimate both the T2 and the B1 maps. We denote this method as Steady-state T2 And Rf Estimation (STARE). STARE offers a new capability to acquire fast 3D dataset for T2 mapping.
|
||
 |
0552.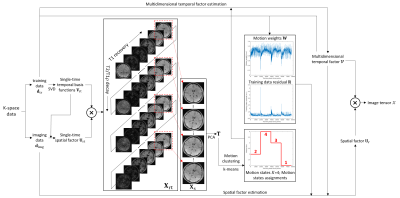 |
Motion-Resolved Brain MRI for Quantitative Multiparametric Mapping
Sen Ma1, Nan Wang1, Zhaoyang Fan1, Yibin Xie1, Debiao Li1, and Anthony G. Christodoulou1
1Biomedical Imaging Research Institute, Cedars-Sinai Medical Center, Los Angeles, CA, United States
We introduce a motion-resolved solution to clinical brain MRI for quantitative multiparametric mapping using Multitasking. We demonstrate that the proposed approach is generalizable to translation, rotation, discrete motion, and periodic motion without explicit need for motion correction or compensation. Both simulation and in vivo results show that the proposed motion-resolved approach produces better image quality with sharp tissue structure and without ghosting/blurring artifacts, which outperforms no motion handling and simple motion removal. The motion-resolved approach yields substantially less RMSE in terms of quantitative mapping accuracy compared to no motion handling and simple motion removal.
|
|
 |
0553.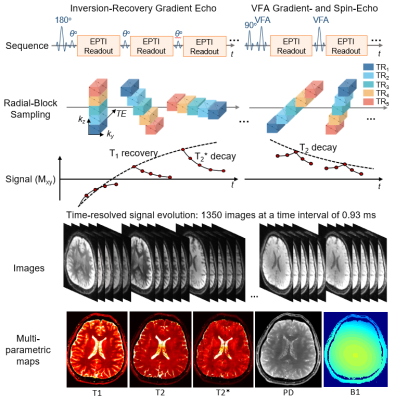 |
Fast and repeatable multi-parametric mapping using 3D Echo-Planar Time-resolved Imaging (3D-EPTI)
Fuyixue Wang1,2, Zijing Dong1,3, Timothy G. Reese1, Lawrence L. Wald1,2, and Kawin Setsompop4,5
1Athinoula A. Martinos Center for Biomedical Imaging, Massachusetts General Hospital, Charlestown, MA, United States, 2Harvard-MIT Health Sciences and Technology, MIT, Cambridge, MA, United States, 3Department of Electrical Engineering and Computer Science, MIT, Cambridge, MA, United States, 4Department of Radiology, Stanford University, Stanford, CA, United States, 5Department of Electrical Engineering, Stanford University, Stanford, CA, United States
3D-EPTI is a recent multi-parametric mapping technique capable of rapid T1,T2,and T2* quantification. In this work, we characterize the repeatability of two optimized 3D-EPTI whole-brain protocols at 1-mm and 0.7-mm isotropic resolutions (3- and 9-minutes), suitable for a range of clinical and neuroscientific applications. Scan-rescan across 5 subjects shows low intra- and inter-subject variabilities in the derived quantitative-metrics across 165 brain-regions using automatic FreeSurfer segmentation. High-repeatability of quantitative measures across cortical depths was demonstrated using the 0.7-mm protocol, indicating its potential for robust-and-repeatable cortical myeloarchitecture investigation. Lastly, synthetic 3D-EPTI images were demonstrated to be in high-agreement with clinical contrast-weighted images.
|
|
 |
0554.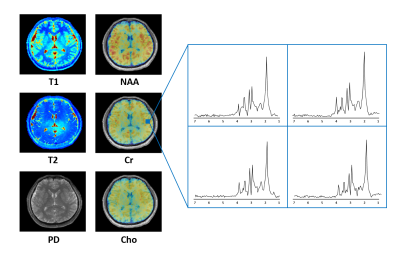 |
Rapid Parametric Mapping Using the Unsuppressed Water Signals in Metabolic Imaging of the Brain
Rong Guo1,2, Yibo Zhao1,2, Yudu Li1,2, Yao Li3, and Zhi-Pei Liang1,2
1Department of Electrical and Computer Engineering, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 2Beckman Institute for Advanced Science and Technology, University of Illinois at Urbana-Champaign, Urbana, IL, United States, 3School of Biomedical Engineering, Shanghai Jiao Tong University, Shanghai, China
Both quantitative MR parametric mapping and MRSI take long scan times. SPICE has recently demonstrated a unique capability for simultaneous metabolic imaging and water imaging. Taking advantage of the unsuppressed water signals acquired using SPICE, we extended the SPICE technique with a new feature for fast parametric mapping. With one-minute extra scan time, T1 and T2 maps at 1.0×1.0×2.0 mm3 resolution were successfully obtained. This new capability enables simultaneous high-resolution parametric mapping and metabolic imaging of the human brain in a total 8-minute scan.
|
|
0555. |
Combined multiparametric high resolution diffusion-relaxometry on 7T (OKAPI)
Jana Hutter1, Raphael Tomi-Tricot2, Jan Sedlacik1, Philippa Bridgen1, Shaihan Malik1, and Joseph V Hajnal1
1Centre for Medical Engineering, King's College London, London, United Kingdom, 2MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom
A multi-parametric quantitative MRI sequence called ZEBRA, which varies diffusion weighting, inversion time (TI) and gradient echo time (TE) by shuffling flexibly diffusion preparation, slice acquisition order, slice spacing and diffusion properties and by adding multiple gradient echo read-outs sharing one diffusion preparation is explored on a 7T scanner in high resolution.
|
||
0556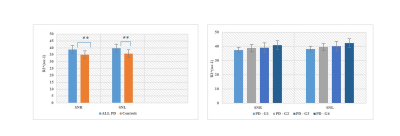 |
Quantifying Brain Iron Deposition in patients with Parkinson’s Disease Using MRI-R2*: A new specific approach developed from a multicenter study Video Permission Withheld
Laila khedher1, Jean Marie Bonny2, Ana Marques3, Marie Vidailhet4, Frédéric Torny5, Luc Defebvre6, Stéphane Thobois7, Elena Moro8, Philippe Remy9, Christian Geny10, Wassilios Meissner11, Solène Frismand12, Anne Doe de Maindreville13,
Jean-Luc Houeto14, Olivier Rascol15, and Franck Durif1,3
1University Clermont Auvergne, Clermont Ferrand, France, 2INRA, UR370 Qualité des Produits Animaux, Saint Genès Champanelle, France, 3CHU Clermont Ferrand, Clermont Ferrand, France, 4Fédération des maladies du système nerveux GH La Pitié Salpêtrière, Paris, France, 5CHU Dupuytren, Service de Neurologie, Limoges, France, 6Hopital Roger Salengro, Service de Neurologie et Pathologie du Mouvement, Lille, France, 7Hopital Pierre Wertheimer, Neurologie C, Lyon, France, 8CHU de Grenoble, Service de Neurologie, Grenoble, France, 9Hopital Henri Mondor, Service de Neurologie, Creteil, France, 10CHRU Montpellier, Service de Neurologie, Montpellier, France, 11CHU Bordeaux, Service de Neurologie, Bordeaux, France, 12Hopital Central-CHU Nancy, Service de Neurologie, Nancy, France, 13Pole Neurologie-Gériatrie, Reims, France, 14CHU de Poitiers, Poitiers, France, 15Centre d’Investigation Clinique CIC 1436, CHU PURPAN-Place du Dr Baylac, Hopital Pierre Paul Riquet, Toulouse, France
Several postmortem studies have shown an accumulation of iron in the substantia nigra (SN) in Parkinson’s disease (PD). The iron concentration can be estimated by MRI via the MRI-R2* mapping. 1, 2 In order to assess the changes in R2* occurring in PD patients compared to healthy controls, a multicenter transversal prospective study was carried out in a large cohort of PD patients (n = 98) going from the early to the late stage of the disease and matched controls (n = 66).
|
||
 |
0557.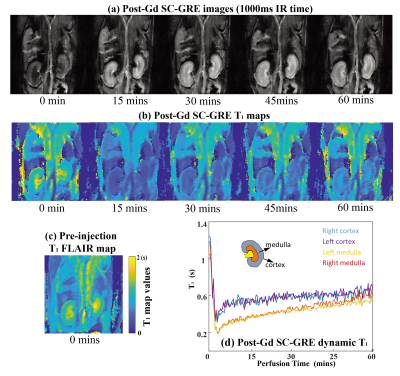 |
Fast T1 mapping and weighting MRI in preclinical and clinical settings using subspace-constrained joint-domains reconstructions
Lingceng Ma1,2, Qingjia Bao1, Ricardo P. Martinho1, Zhong Chen2, and Lucio Frydman1
1Department of Chemical and Biological Physics, Weizmann Institute of Science, Rehovot, Israel, 2Department of Electronic Science, Xiamen University, Xiamen, China
Fast T1 mapping methods based on subspace-constrained reconstructions of jointly sparsed-sample domains, are proposed and shown to efficiently deliver maps with either multiple T1 contrasts or T1 values with ≈ 50-100× accelerations. Both single-shot and multi-shot implementations were developed, incorporating random-sampling inversion recovery (IR) as well as variable-TR multi-shot gradient echo (GRE) and spatiotemporally encoded (SPEN) sequences. In vivo human brain scans confirmed the efficiency of this method. Preclinical scans on kidneys and on tumor-implanted animals subject to dynamic contrast-enhanced T1 mapping, also demonstrate the proposed method's advantages for functional and pathological diagnoses.
|
|
0558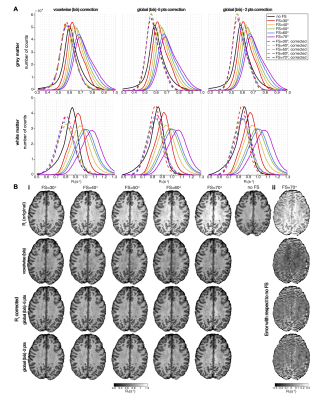 |
Quantitative T1 mapping by multi-slice multi-shot inversion recovery EPI: correction of fat suppression MT effects. Video Permission Withheld
Rosa Sanchez Panchuelo1, Olivier Mougin1, Robert Turner1,2, and Susan Francis1,3
1Sir Peter Mansfield Imaging Centre, UP, University of Nottingham, Nottingham, United Kingdom, 2Max Planck Institute for Human Cognitive and Brain Sciences, Leibzig, Germany, 3NIHR Nottingham Biomedical Research, University of Nottingham, Nottingham, United Kingdom
Multi-slice inversion–recovery EPI (MS-IR-EPI) combined with slice order shifting across multiple acquisitions can provide a fast method for high spatial resolution T1 mapping. However, magnetization transfer (MT) effects of spectrally-selective fat-suppression pulses used in in-vivo imaging shorten measured T1-values. Here we model the effect of fat-suppression pulses on measured T1 and use this model to remove the MT contribution, improving the accuracy of T1 quantification. MT-corrected high spatial resolution T1 maps of the human brain generated with MS-IR-EPI at 7T are compared with those generated with the widely implemented MP2RAGE sequence and standard single slice IR-EPI.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.