Oral
Systems Engineering II
ISMRM & SMRT Annual Meeting • 15-20 May 2021

| Concurrent 2 | 18:00 - 20:00 | Moderators: Hui Han & Kalina Jordan |
 |
0619.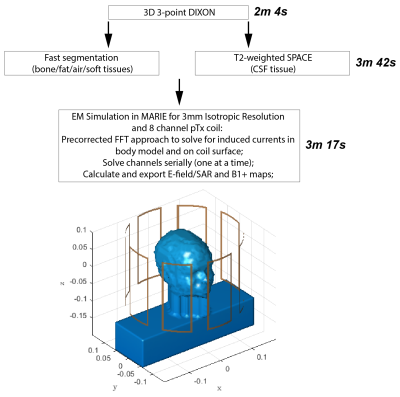 |
Approaching Real-Time Patient-Specific SAR Calculation for Parallel Transmission at 7 Tesla
Eugene Milshteyn1,2, Georgy Guryev3, Angel Torrado-Carvajal1,2,4, Jacob K. White3, Lawrence L. Wald1,2,5, and Bastien Guerin1,2
1Athinoula A. Martinos Center for Biomedical Imaging, Charlestown, MA, United States, 2Harvard Medical School, Boston, MA, United States, 3Dept. of Electrical Engineering and Computer Science, Massachusetts Institute of Technology, Cambridge, MA, United States, 4Medical Image Analysis and Biometry Laboratory, Universidad Rey Juan Carlos, Madrid, Spain, 5Harvard-MIT Division of Health Sciences and Technology, Cambridge, MA, United States
Current parallel transmission protocols have conservative safety limits due to offline simulations of generic body models. Instead, a personalized medicine approach should be used, whereby the patient-specific SAR is calculated as the patient lies on the table. In this way, more accurate, and hopefully less conservative safety limits can be employed. In this study, we develop a fast methodology for patient-specific SAR calculations with an 8 channel pTx head coil at 7T. We show a real-time approach in 6 volunteers for scanning the patient, segmenting the body, and performing an electromagnetic simulation in order to generate the local SAR maps.
|
|
 |
0620.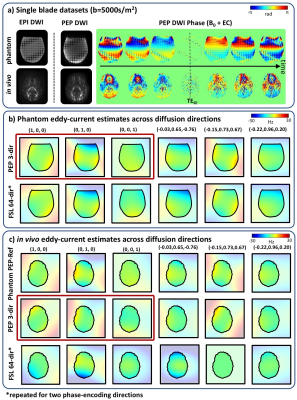 |
Rapid calibration scan for estimating temporally-varying eddy currents in diffusion imaging using a time-resolved PEPTIDE imaging approach
Merlin J Fair1 and Kawin Setsompop1,2
1Radiological Sciences Laboratory, Stanford University, Stanford, CA, United States, 2Electrical Engineering, Stanford University, Stanford, CA, United States
A rapid calibration scan for estimating eddy-currents in diffusion acquisitions was developed using the time-resolved PEPTIDE imaging approach. This calibration scan estimates temporally-varying eddy-current fields across three principal diffusion-directions in <30s, with estimates of eddy-fields across other directions derived through a linear model. The accuracy of this method was validated in simulation, phantom and in-vivo experiments. In a high-SNR diffusion phantom, estimated eddy-fields closely match that of “FSL-eddy” on a large blip-up and blip-down 64-direction EPI dataset. For in-vivo data at b=5000s/mm2, estimates from PEPTIDE-based calibration was able to maintain high-accuracy estimation of the eddy-current field despite low SNR.
|
|
0621.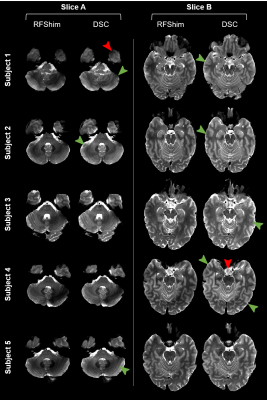 |
Fully Integrated Scanner Implementation of Direct Signal Control for 2D T2-Weighted TSE at Ultra-High Field
Raphael Tomi-Tricot1,2,3, Jan Sedlacik2,3, Jonathan Endres4, Juergen Herrler5, Patrick Liebig6, Rene Gumbrecht6, Dieter Ritter6, Tom Wilkinson2,3, Pip Bridgen7, Sharon Giles7, Armin M. Nagel4, Joseph V. Hajnal2,3, Radhouene Neji1,2,
and Shaihan J. Malik2,3
1MR Research Collaborations, Siemens Healthcare Limited, Frimley, United Kingdom, 2Biomedical Engineering Department, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 3Centre for the Developing Brain, School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom, 4Institute of Radiology, University Hospital Erlangen, Erlangen, Germany, 5Institute of Neuroradiology, University Hospital Erlangen, Erlangen, Germany, 6Siemens Healthcare GmbH, Erlangen, Germany, 7School of Biomedical Engineering and Imaging Sciences, King's College London, London, United Kingdom
Direct Signal Control (DSC) uses parallel transmission (pTx) with more flexibility than conventional static RF shimming to tackle RF inhomogeneity at ultra-high field in fast spin echo (FSE) sequences by varying complex weights of successive RF pulses independently along the refocusing train. Also, unlike other dynamic pTx methods, it preserves RF pulse properties and sequence timing. This work demonstrates the applicability of DSC in routine conditions for neuroimaging, with minimal workflow disruption. In-vivo T2-weighted FSE results exhibit higher signal and better homogeneity when using DSC over RF shimming, while explicitly ensuring safe operation.
|
||
 |
0622.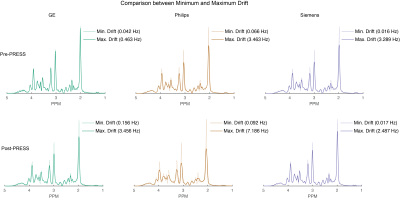 |
Frequency Drift in MR Spectroscopy: An 87-scanner 3T Phantom Study
Steve C.N. Hui1,2, Mark Mikkelsen1,2, Helge J. Zöllner1,2, Vishwadeep Ahluwalia3, Sarael Alcauter4, Laima Baltusis5, Deborah A. Barany6, Laura R. Barlow7, Robert Becker8, Jeffrey I. Berman9, Adam Berrington10, Pallab K. Bhattacharyya11, Jakob Udby Blicher12,
Wolfgang Bogner13, Mark S. Brown14, Vince D. Calhoun15, Ryan Castillo16, Kim M. Cecil17, Yeo Bi Choi18, Winnie C.W. Chu19, William T. Clarke20, Alexander R. Craven21, Koen Cuypers22, Michael Dacko23, Camilo de la Fuente-Sandoval24, Patricia Desmond25,
Aleksandra Domagalik26, Julien Dumont27, Niall W. Duncan28, Ulrike Dydak29, Katherine Dyke30, David A. Edmondson17, Gabriele Ende8, Lars Ersland31, C. John Evans32, Alan S. R. Fermin33, Antonio Ferretti34, Ariane Fillmer35, Tao Gong36,
Ian Greenhouse37, James T. Grist38, Meng Gu39, Ashley D. Harris40, Katarzyna Hat41, Stefanie Heba42, Eva Heckova13, John P. Hegarty II43, Kirstin-Friederike Heise44, Aaron Jacobson45, Jacobus F.A. Jansen46, Christopher W. Jenkins47, Stephen J. Johnston48,
Christoph Juchem49, Alayar Kangarlu50, Adam B. Kerr5, Karl Landheer51, Thomas Lange52, Phil Lee53, Swati Rane Levendovszky54, Catherine Limperopoulos55, Feng Liu56, William Lloyd57, David J. Lythgoe58, Maro G. Machizawa59, Erin L. MacMillan7,
Richard J. Maddock60, Andrei V. Manzhurtsev61, María L. Martinez-Gudino62, Jack J. Miller63, Heline Mirzakhanian64, Paul G. Mullins65, Jamie Near66, Wibeke Nordhøy67, Georg Oeltzschner1,2, Raul Osorio62, Maria C.G. Otaduy68, Erick H. Pasaye4, Ronald Peeters69,
Scott J. Peltier70, Ulrich Pilatus71, Nenad Polomac71, Eric C. Porges72, Subechhya Pradhan55, James Joseph Prisciandaro73, Nick Puts74, Caroline D. Rae75, Francisco Reyes-Madrigal76, Timothy P.L. Roberts9, Caroline E. Robertson77, Muhammad G. Saleh78, Jens T. Rosenberg79,
Diana-Georgiana Rotaru58, O'Gorman Tuura L. Ruth80, Kristian Sandberg12, Ryan Sangill81, Keith Schembri82, Anouk Schrantee83, Natalia A. Semenova84, Debra Singel85, Rouslan Sitnikov86, Jolinda Smith87, Yulu Song36, Craig Stark88, Diederick Stoffers89,
Stephan P. Swinnen44, Costin Tanase60, Sofie Tapper1,2, Martin Tegenthoff42, Thomas Thiel90, Marc Thioux91, Peter Truong92, Pim van Dijk91, Nolan Vella82, Rishma Vidyasagar93, Andrej Vovk94, Guangbin Wang36, Lars T. Westle67,
Timothy K. Wilbur54, William R. Willoughby95, Martin Wilson96, Hans-Jörg Wittsack97, Adam J. Woods98, Yen-Chien Wu99, Junqian Xu100, Maria Yanez Lopez101, David K.W. Yeung19, Qun Zhao102, Xiaopeng Zhou29, Gasper Zupan94, and Richard A.E. Edden1,2
1Russell H. Morgan Department of Radiology and Radiological Science, The Johns Hopkins University School of Medicine, Baltimore, MD, United States, 2F.M. Kirby Research Center for Functional Brain Imaging, Kennedy Krieger Institute, Baltimore, MD, United States, 3GSU/GT Center for Advanced Brain Imaging, Georgia Institute of Technology, Atlanta, GA, United States, 4Instituto de Neurobiología, Universidad Nacional Autónoma de México, Queretaro, Mexico, 5Center for Cognitive and Neurobiological Imaging, Stanford University, Stanford, CA, United States, 6Kinesiology, University of Georgia, Athens, GA, United States, 7Department of Radiology, The University of British Columbia, Vancouver, BC, Canada, 8Center for Innovative Psychiatry and Psychotherpay Research, Department Neuroimaging, Central Institute of Mental Health, Medical Faculty Mannheim, Heidelberg University, Mannheim, Germany, 9Department of Radiology, Children's Hospital of Philadelphia, Philadelphia, PA, United States, 10Sir Peter Mansfield Imaging Centre, School of Physics and Astronomy, University of Nottingham, Nottingham, United Kingdom, 11Imaging Institute, The Cleveland Clinic, Cleveland, OH, United States, 12Center of Functionally Integrative Neuroscience, Aarhus University, Aarhus, Denmark, 13Department of Biomedical Imaging and Image-guided Therapy, High-Field MR Center, Medical University of Vienna, Vienna, Austria, 14Department of Radiology, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 15Tri-Institutional Center for Translational Research in Neuroimaging and Data Science(TReNDS), Georgia State University, Georgia Institute of Technology, and Emory University, Atlanta, GA, United States, 16Neuroscience Research AustraliaNeuRA Imaging, Randwick, Australia, 17Department of Radiology, Cincinnati Children's Hospital Medical Center, Cincinnati, OH, United States, 18Psychological and Brain Sciences, Dartmouth College, Hanover, NH, United States, 19Department of Imaging & Interventional Radiology, The Chinese University of Hong Kong, Hong Kong, Hong Kong, 20Wellcome Centre for Integrative Neuroimaging, NDCN, University of Oxford, Oxford, United Kingdom, 21Department of Biological and Medical Psychology, University of Bergen, Haukeland University Hospital, Bergen, Norway, 22REVAL Rehabilitation Research Institute (REVAL), Hasselt University, Diepenbeek, Belgium, 23Department of Radiology, Medical Physics, Medical Center - University of Freiburg, Faculty of Medicine, University of Freiburg, Freiburg, Germany, 24Laboratory of Experimental Psychiatry & Neuropsychiatry Department, Instituto Nacional de Neurología y Neurocirugía, Mexico City, Mexico, 25Department of Radiology, University of Melbourne/ Royal Melbourne Hospital, Melbourne, Australia, 26Malopolska Centre of Biotechnology, Jagiellonian University, Krakow, Poland, 27Clinical Imaging Core Facility, CI2C Lille, Lille, France, 28Graduate Institute of Mind, Brain and Consciousness, Taipei Medical University, Taipei, Taiwan, 29School of Health Sciences, Purdue University, West Lafayette, IN, United States, 30School of Psychology, University of Nottingham, Nottingham, United Kingdom, 31Department of Clinical Engineering, University of Bergen, Haukeland University Hospital, Bergen, Norway, 32CUBRIC, Cardiff University, Cardiff, United Kingdom, 33Center for Brain, Mind and Kansei Sciences Research, Hiroshima University, Hiroshima, Japan, 34Neuroscience, Imaging and Clinical Sciences, University "G. d'Annunzio" of Chieti-Pescara, Chieti, Italy, 35Physikalisch-Technische Bundesanstalt (PTB), Braunschweig und Berlin, Germany, 36Department of Imaging and Nuclear Medicine, Shandong Medical Imaging Research Institute, Shandong University, Jinan, China, 37Human Physiology, University of Oregon, Eugene, OR, United States, 38Physiology, Anatomy, and Genetics/ Oxford Centre for Magnetic Resonance, The University of Oxford / Department of Radiology, The Churchill Hospital, The University of Oxford, Oxford, United Kingdom, 39Department of Radiology, Stanford University, Stanford, CA, United States, 40Department of Radiology, University of Calgary, Calgary, AB, Canada, 41Institute of Psychology, Jagiellonian University, Krakow, Poland, 42Department of Neurology, BG University Hospital Bergmannsheil, Bochum, Germany, 43Psychiary & Behavioral Sciences, Stanford University, Stanford, CA, United States, 44Department of Movement Sciences, KU Leuven, Leuven, Belgium, 45Department of Radiology, University of California San Diego, San Diego, CA, United States, 46Department of Radiology and Nuclear Medicine, Maastricht University Medical Center, Maastricht, Netherlands, 47CUBRIC, Cardiff university, Cardiff, United Kingdom, 48Psychology Dept. / Clinical Imaging Facility, Swansea University, Swansea, United Kingdom, 49Biomedical Engineering and Radiology, Columbia University, New York City, NY, United States, 50Psychiatry, Columbia University Irving Medical Center/New York State Psychiatric Institute, New York City, NY, United States, 51Biomedical Engineering, Columbia University, New York City, NY, United States, 52Department of Radiology, Medical Physics, University of Freiburg, Freiburg, Germany, 53Department of Radiology, University of Kansas Medical Center, Kansas, KS, United States, 54Department of Radiology, University of Washington, Seattle, WA, United States, 55Developing Brain Institute, Diagnostic Imaging and Radiology, Children's National Hospital, Washington, DC, United States, 56Department of Psychiatry, Columbia University Irving Medical Center/New York State Psychiatric Institute, New York, NY, United States, 57Division of Informatics, Imaging & Data Sciences, University of Manchester, Manchester, United Kingdom, 58Department of Neuroimaging, King's College London, London, United Kingdom, 59Center for Brain, Mind and KANSEI Sciences Research, Hiroshima University, Hiroshima, Japan, 60Psychiatry and Behavioral Sciences, University of California Davis, Imaging Research Center, Davis, CA, United States, 61Department of Radiology, Clinical and Research Institute of Emergency Pediatric Surgery and Trauma, Moscow, Russian Federation, 62Imágenes Cerebrales, Instituto Nacional de Psiquiatría Ramón de la Fuente, Mexico City, Mexico, 63Department of Physics, University of Oxford, Oxford, United Kingdom, 64Department of Psychiatry, University of California San Diego, San Diego, CA, United States, 65Department of Psychology, Bangor University, Bangor, United Kingdom, 66Douglas Mental Health University Institute and Department of Psychiatry, McGill University, Montreal, QC, Canada, 67Department of Diagnostic Physics, Division of Radiology and Nuclear Medicine, Oslo University Hospital, Oslo, Norway, 68LIM44, Instituto e Departamento de Radiologia, Faculdade de Medicina, Universidade de Sao Paulo, Sao Paulo, Brazil, 69Department of Imaging & Pathology, Department of Radiology, University Hospitals Leuven, KU Leuven, Leuven, Belgium, 70Functional MRI Laboratory, University of Michigan, Ann Arbor, MI, United States, 71Institute of Neuroradiology, Goethe-University Frankfurt, Frankfurt, Germany, 72Center for Cognitive Aging and Memory, McKnight Brain Institute, Department of Clinical and Health Psychology, College of Public Health and Health Professions, University of Florida, Gainesville, FL, United States, 73Psychiatry and Behavioral Sciences, Medical University of South Carolina, Charleston, SC, United States, 74Forensic & Neurodevelopmental Sciences, King's College London, London, United Kingdom, 75NeuRA Imaging, Neuroscience Research Australia, Randwick, Australia, 76Laboratory of Experimental Psychiatry, Instituto Nacional de Neurología y Neurocirugía, Mexico City, Mexico, 77Department of Psychological and Brain Sciences, Dartmouth College, Hanover, NH, United States, 78Department of Diagnostic Radiology and Nuclear Medicine, University of Maryland School of Medicine, Baltimore, MD, United States, 79McKnight Brain Institute, AMRIS, University of Florida, Gainesville, FL, United States, 80Center for MR Research, University Children's Hospital, Zurich, Switzerland, 81Center of Functionally Integrative Neuroscience, Aarhus University Hospital, Aarhus, Denmark, 82Medical Physics, Mater Dei Hospital, Imsida, Malta, 83Department of Radiology and Nuclear Medicine, Amsterdam University Medical Center, University of Amsterdam, Amsterdam, Netherlands, 84504, Emanuel Institute of Biochemical Physics of the Russian Academy of Sciences, Moscow, Russian Federation, 85Psychiatry, University of Colorado Anschutz Medical Campus, Aurora, CO, United States, 86Clinical Neuroscience, MRI Centre, Karolinska Institutet, Clinical Neuroscience, MRI Centre, Sweden, 87Lewis Center for Neuroimaging, University of Oregon, Eugene, OR, United States, 88Department of Neurobiology and Behavior, Facility for Imaging and Brain Research (FIBRE) & Campus Center for Neuroimaging (CCNI), University of California, Irvine, Irvine, CA, United States, 89Spinoza Centre for Neuroimaging, Royal Netherlands Academy of Arts and Sciences, Amsterdam, Netherlands, 90Institute of Clinical Neuroscience and Medical Psychology, University Dusseldorf, Medical Faculty, Düsseldorf, Germany, 91Otorhinolaryngology, Head and Neck Surgery, University of Groningen, University Medical Center Groningen, Groningen, Netherlands, 92Brain Health Imaging Centre, Centre for Addiction and Mental Health, Toronto, ON, Canada, 93Melbourne Dementia Research Centre, Florey Institute of Neurosciences and Mental Health, Melbourne, Australia, 94Faculty of Medicine, University of Ljubljana, Ljubljana, Slovenia, 95Department of Radiology, University of Alabama at Birmingham, Birmingham, AL, United States, 96Centre for Human Brain Health, University of Birmingham, Birmingham, United Kingdom, 97Department of Diagnostic and Interventional Radiology, University Dusseldorf, Medical Faculty, Düsseldorf, Germany, 98Center for Cognitive Aging and Memory, McKnight Brain Institute, Department of Clinical and Health Psychology, College of Public Health and Health Professions. Department of Neuroscience, College of Medicine, University of Florida, Gainesville, FL, United States, 99Department of Radiology, TMU-Shuang Ho Hospital, New Taipei City, Taiwan, 100Department of Radiology and Psychiatry, Baylor College of Medicine, Houston, TX, United States, 101Perinatal Imaging & Health, King's College London, London, United Kingdom, 102Bioimaging Research Center, University of Georgia, Athens, GA, United States
This project aimed to examine the relationship between gradient-induced heating and field drift on a large sample of MRI scanners. A standardized phantom protocol was established, and spectroscopy was performed before and after running 10 minutes of echo-planar imaging (EPI). MRS data were acquired from 87 scanners. The frequency drift trace was extracted by modeling the water signal in each transient. Drift rates of up to 1.3 Hz/minute were seen before EPI, and 4 Hz/minute after. This dataset will allow sites to benchmark scanner drift, for consideration in planning research protocol order and examine the need for real-time field-frequency locking.
|
|
 |
0623. |
A 64-Channel Brain Array Coil with an Integrated 16-Channel Field Monitoring System for 3T MRI
Mirsad Mahmutovic1, Alina Scholz1, Nicolas Kutscha1, Markus W. May1, Torsten Schlumm2, Roland Müller2, Kerrin Pine2, Luke J. Edwards2, Nikolaus Weiskopf2,3, David O. Brunner4, Harald E. Möller2, and Boris Keil1
1Institute of Medical Physics and Radiation Protection, TH Mittelhessen University of Applied Sciences, Giessen, Germany, 2Max Planck Institute for Human Cognitive and Brain Sciences, Leipzig, Germany, 3Felix Bloch Institute for Solid State Physics, Faculty of Physics and Earth Sciences, Leipzig University, Leipzig, Germany, 4Skope Magnetic Resonance Technologies AG, Zurich, Switzerland
Combining highly parallel array coils, magnetic field monitoring, and high gradient strength provide a complementary approach to enhance diffusion-weighted imaging (DWI). A 64-channel receive brain array with an incorporated 16-channel field camera system was developed to be used with 300 mT/m gradients from the 3T Connectom scanner. The increased signal-to-noise ratio (SNR) and implemented parallelism was highly beneficial, and will improve SNR-starved high-b value DWI acquisitions, while the magnetic field monitoring data successfully corrected the images from blurring, aliasing and distortions.
|
|
 |
0624.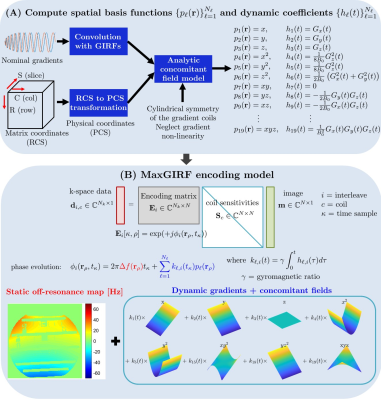 |
MaxGIRF: Image Reconstruction Incorporating Maxwell Fields and Gradient Impulse Response Function Distortion
Nam G. Lee1, Rajiv Ramasawmy2, Adrienne E. Campbell-Washburn2, and Krishna S. Nayak1,3
1Biomedical Engineering, University of Southern California, Los Angeles, CA, United States, 2Cardiovascular Branch, Division of Intramural Research, National Heart, Lung, and Blood Institute, National Institutes of Health, Bethesda, MD, United States, 3Ming Hsieh Department of Electrical and Computer Engineering, University of Southern California, Los Angeles, CA, United States
Non-Cartesian imaging can suffer from local blurring caused by concomitant fields and off-resonance. Concomitant fields are especially problematic when using prolonged non-Cartesian readouts with high gradient amplitudes at lower field strengths. We present a new reconstruction method, denoted MaxGIRF, for non-Cartesian imaging that corrects concomitant fields and trajectory errors without specialized hardware. The proposed method utilizes gradient impulse response functions to predict gradients waveforms which are in-turn used to estimate concomitant fields with analytic expressions. Image artifacts were successfully mitigated by the proposed method from 2D SE spiral imaging of the human brain acquired on a prototype 0.55T MRI system.
|
|
0625.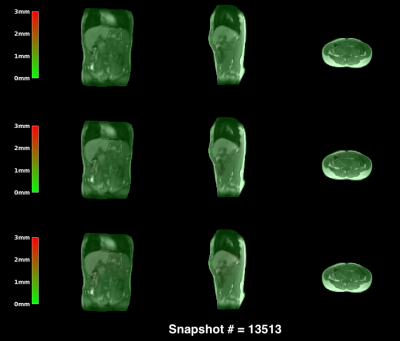 |
Joint 3D motion-field and uncertainty estimation at 67Hz on an MR-LINAC
Niek RF Huttinga1, Tom Bruijnen1, Cornelis AT van den Berg1, and Alessandro Sbrizzi1
1Department of Radiotherapy, Computational Imaging Group for MR therapy & Diagnostics, University Medical Center Utrecht, Utrecht, Netherlands
We present a probabilistic framework to perform simultaneous real-time 3D motion estimation and uncertainty quantification. We extend our preliminary work to a realistic prospective in-vivo setting, and demonstrate it on an MR-LINAC. The acquisition+processing time for 3D motion-fields is around 15ms, yielding a 67Hz frame-rate. Results indicate high quality predictions, and uncertainty estimates that could be used for real-time quality assurance during MR-guided radiotherapy on an MR-LINAC.
|
||
 |
0626.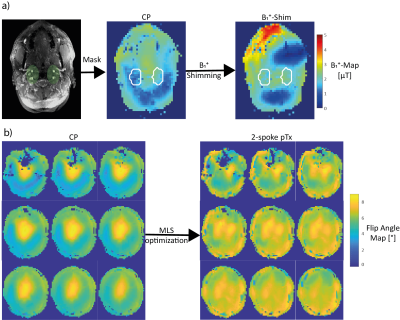 |
Impact of B1+-Shimming and 2-spoke pTx on 4D Angiography at 7T
Christian R. Meixner1, Sebastian Schmitter2,3, Jürgen Herrler4, Arnd Dörfler4, Michael Uder1, and Armin M. Nagel1,3
1Institute of Radiology, University Hospital Erlangen, Friedrich-Alexander-Universität Erlangen-Nürnberg, Erlangen, Germany, 2Physikalisch-Technische Bundesanstalt (PTB), Braunschweig und Berlin, Germany, 3Division of Medical Physics in Radiology, German Cancer Research Center (DKFZ), Heidelberg, Germany, 4Institute of Neuro-Radiology, Friedrich-Alexander Universität Erlangen-Nürnberg, Erlangen, Germany
4D-angiography exploiting pseudo-continuous arterial spin labeling at 7T suffers from specific absorption rate constraints, low B1+ efficiency for the labeling and B1+ inhomogeneity in the readout. In this work, we propose a B1+-phase shim trading B1+ homogeneity and transmit efficiency for the labeling combined with a dynamic transmission 2-spoke excitation readout. In volunteer measurements, the proposed approach outperformed the standard circular polarized mode by an increased vessel intensity and more vessel conspicuity.
|
|
 |
0627.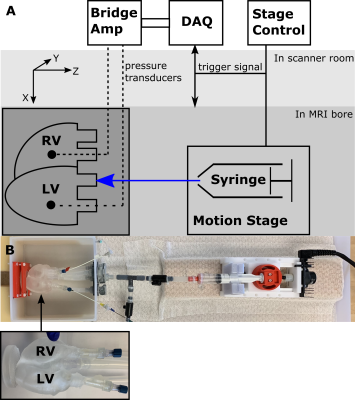 |
System for Validating MRI-based Myocardial Stiffness Estimation Techniques Using 3D-Printed Heart Phantoms
Fikunwa O. Kolawole1,2, Tyler Edward Cork2,3,4, Michael Loecher2,3, Judith Zimmermann3,5, Seraina A. Dual3, Marc E. Levenston1,3, and Daniel B. Ennis2,3
1Mechanical Engineering, Stanford University, Stanford, CA, United States, 2Radiology, Veterans Administration Health Care System, Palo Alto, CA, United States, 3Radiology, Stanford University, Stanford, CA, United States, 4Bioengineering, Stanford University, Stanford, CA, United States, 5Computer Science, Technical University of Munich, Garching, Germany
Cardiac MRI and finite element based techniques can be used to obtain subject-specific myocardial material properties. Verifying the accuracy and precision of these techniques requires overcoming the challenge of obtaining ground-truth in vivo myocardial stiffness estimates. This work presents a highly controlled in vitro diastolic filling setup incorporating a 3D-printed heart phantom developed with myocardial tissue-mimicking material of known mechanical and MRI properties. The setup enables acquisition of the data needed to estimate myocardial stiffness in computational models: phantom geometry, loading pressures, boundary conditions, and filling strains. This setup is designed to enable extensive validation of myocardial stiffness estimation frameworks.
|
|
0628. |
Disposable Point-of-care Portable Perfusion Phantom for Accurate Quantitative DCE-MRI
Martin Dawson Holland1, Andres Morales1, Sean Simmons2, Brandon Smith1, Samuel R Misko1, Roy P Koomullil1, Junzhong Xu3, David A Hormuth, II4, Junzhong Xu3, Thomas E Yankeelov4, and Harrison Kim1
1University of Alabama at Birmingham, Birmingham, AL, United States, 2Objective Design, Birmingham, AL, United States, 3Vanderbilt University Medical Center, Nashville, TN, United States, 4University of Texas, Austin, TX, United States
A new point-of-care portable perfusion phantom was developed to reduce inter- and intra-scanner variability of quantitative dynamic contrast enhanced magnetic resonance imaging (DCE-MRI). This device is disposable, easily operable, and conveniently deliverable for widespread, routine clinical use. As this device has high repeatability (intraclass correlation coefficient = 0.997), it can be utilized to improve the accuracy of quantitative DCE-MRI based analysis of many diseases including cancer.
|
The International Society for Magnetic Resonance in Medicine is accredited by the Accreditation Council for Continuing Medical Education to provide continuing medical education for physicians.